Sensing and tactile artificial muscles from reactive materials
- PMID: 22319265
- PMCID: PMC3274195
- DOI: 10.3390/s100402638
Sensing and tactile artificial muscles from reactive materials
Abstract
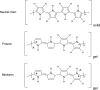
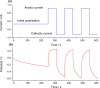
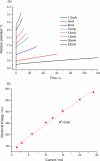
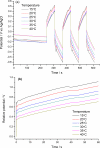
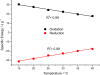
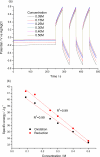
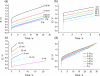
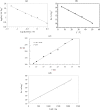
Films of conducting polymers can be oxidized and reduced in a reversible way. Any intermediate oxidation state determines an electrochemical equilibrium. Chemical or physical variables acting on the film may modify the equilibrium potential, so that the film acts as a sensor of the variable. The working potential of polypyrrole/DBSA (Dodecylbenzenesulfonic acid) films, oxidized or reduced under constant currents, changes as a function of the working conditions: electrolyte concentration, temperature or mechanical stress. During oxidation, the reactive material is a sensor of the ambient, the consumed electrical energy being the sensing magnitude. Devices based on any of the electrochemical properties of conducting polymers must act simultaneously as sensors of the working conditions. Artificial muscles, as electrochemical actuators constituted by reactive materials, respond to the ambient conditions during actuation. In this way, they can be used as actuators, sensing the surrounding conditions during actuation. Actuating and sensing signals are simultaneously included by the same two connecting wires.
Keywords: actuators; artificial muscles; conducting polymers; reactive materials; sensing actuators; sensors; tactile muscles.
Figures












Similar articles
-
Biomimetic dual sensing-actuators: theoretical description. Sensing electrolyte concentration and driving current.J Phys Chem B. 2012 Aug 2;116(30):9223-30. doi: 10.1021/jp302931k. Epub 2012 Jul 13. J Phys Chem B. 2012. PMID: 22735073
-
Biomimetic dual sensing-actuators based on conducting polymers. Galvanostatic theoretical model for actuators sensing temperature.J Phys Chem B. 2012 May 3;116(17):5279-90. doi: 10.1021/jp300290s. Epub 2012 Apr 19. J Phys Chem B. 2012. PMID: 22455612
-
Reactive conducting polymers as actuating sensors and tactile muscles.Bioinspir Biomim. 2008 Sep;3(3):035004. doi: 10.1088/1748-3182/3/3/035004. Epub 2008 Jul 31. Bioinspir Biomim. 2008. PMID: 18667760
-
Conjugated Polymer Actuators and Devices: Progress and Opportunities.Adv Mater. 2019 May;31(22):e1808210. doi: 10.1002/adma.201808210. Epub 2019 Mar 25. Adv Mater. 2019. PMID: 30907471 Review.
-
Carbon nanotube and graphene-based bioinspired electrochemical actuators.Adv Mater. 2014 Feb;26(7):1025-43. doi: 10.1002/adma.201303432. Epub 2013 Dec 12. Adv Mater. 2014. PMID: 24338697 Review.
Cited by
-
Antagonist Concepts of Polypyrrole Actuators: Bending Hybrid Actuator and Mirrored Trilayer Linear Actuator.Polymers (Basel). 2021 Mar 11;13(6):861. doi: 10.3390/polym13060861. Polymers (Basel). 2021. PMID: 33799659 Free PMC article.
-
Electroactive macromolecular motors as model materials of ectotherm muscles.RSC Adv. 2021 Jun 17;11(35):21489-21506. doi: 10.1039/d1ra02573b. eCollection 2021 Jun 15. RSC Adv. 2021. PMID: 35478837 Free PMC article. Review.
-
Technologies and Sensors for Artificial Muscles in Rehabilitation.Sensors (Basel). 2024 Nov 26;24(23):7532. doi: 10.3390/s24237532. Sensors (Basel). 2024. PMID: 39686069 Free PMC article. Review.
-
Simultaneous Sensing and Actuating Capabilities of a Triple-Layer Biomimetic Muscle for Soft Robotics.Sensors (Basel). 2023 Nov 12;23(22):9132. doi: 10.3390/s23229132. Sensors (Basel). 2023. PMID: 38005519 Free PMC article.
-
High-Performance Dopamine-Based Supramolecular Bio-Adhesives.Macromol Rapid Commun. 2024 Dec;45(23):e2400345. doi: 10.1002/marc.202400345. Epub 2024 Jun 6. Macromol Rapid Commun. 2024. PMID: 38760014 Free PMC article.
References
-
- Stryer L., Berg J.M., Tymoczko J.L. Biochemistry. 4th ed. W.H. Freeman & Company; New York, NY, USA: 1995.
-
- Otero T.F., Rodriguez J. Electrochemomechanical and electrochemopositioning devices: artifcial muscles. In: Aldissi M., editor. Intrinsically Conducting Polymers: An Emerging Technology. Kluwer; Dordrecht, The Netherlands: 1993. pp. 179–190.
-
- Otero T.F. Conducting polymers, electrochemistry, and biomimicking processes. In: Bockris J.O.M., White R.E., Conway B.E., editors. Modern Aspects of Electrochemistry. Kluwer Academic/Plenum Publisher; New York, NY, USA: 1999. pp. 307–434.
-
- Otero T.F. Electrochemomechanical devices based on conducting polymers. In: de Rossi D., Osada Y., editors. Polymer Sensors and Actuators; Berlin, Germany: Springer; 2000. pp. 295–323.
-
- Otero T.F. Biomimicking materials with smart polymers. In: Elices M., Cahn R.W., editors. Structural Biological Materials. Design and Structure-Properties Relationships. Pergamon Materials Series; Amsterdam, the Netherlands: 2000. pp. 187–220.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

