Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death
- PMID: 22319448
- PMCID: PMC3271072
- DOI: 10.1371/journal.ppat.1002507
Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death
Abstract
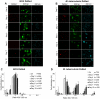
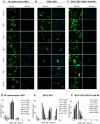
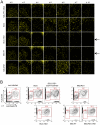
Survival within macrophages is a central feature of Mycobacterium tuberculosis pathogenesis. Despite significant advances in identifying new immunological parameters associated with mycobacterial disease, some basic questions on the intracellular fate of the causative agent of human tuberculosis in antigen-presenting cells are still under debate. To get novel insights into this matter, we used a single-cell fluorescence resonance energy transfer (FRET)-based method to investigate the potential cytosolic access of M. tuberculosis and the resulting cellular consequences in an unbiased, quantitative way. Analysis of thousands of THP-1 macrophages infected with selected wild-type or mutant strains of the M. tuberculosis complex unambiguously showed that M. tuberculosis induced a change in the FRET signal after 3 to 4 days of infection, indicating phagolysosomal rupture and cytosolic access. These effects were not seen for the strains M. tuberculosisΔRD1 or BCG, both lacking the ESX-1 secreted protein ESAT-6, which reportedly shows membrane-lysing properties. Complementation of these strains with the ESX-1 secretion system of M. tuberculosis restored the ability to cause phagolysosomal rupture. In addition, control experiments with the fish pathogen Mycobacterium marinum showed phagolysosomal translocation only for ESX-1 intact strains, further validating our experimental approach. Most importantly, for M. tuberculosis as well as for M. marinum we observed that phagolysosomal rupture was followed by necrotic cell death of the infected macrophages, whereas ESX-1 deletion- or truncation-mutants that remained enclosed within phagolysosomal compartments did not induce such cytotoxicity. Hence, we provide a novel mechanism how ESX-1 competent, virulent M. tuberculosis and M. marinum strains induce host cell death and thereby escape innate host defenses and favor their spread to new cells. In this respect, our results also open new research directions in relation with the extracellular localization of M. tuberculosis inside necrotic lesions that can now be tackled from a completely new perspective.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
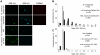
Comment in
-
Bacterial pathogenesis: TB blurs the lines.Nat Rev Microbiol. 2012 Jun 15;10(7):442. doi: 10.1038/nrmicro2825. Nat Rev Microbiol. 2012. PMID: 22699958 No abstract available.
References
-
- Kumar D, Rao KV. Regulation between survival, persistence, and elimination of intracellular mycobacteria: a nested equilibrium of delicate balances. Microbes Infect. 2011;13:121–133. - PubMed
-
- Koul A, Herget T, Klebl B, Ullrich A. Interplay between mycobacteria and host signalling pathways. Nat Rev Microbiol. 2004;2:189–202. - PubMed
-
- Vergne I, Chua J, Singh SB, Deretic V. Cell biology of Mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. - PubMed
-
- Russell DG. Phagosomes, fatty acids and tuberculosis. Nat Cell Biol. 2003;5:776–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

