The mitochondrial chaperone protein TRAP1 mitigates α-Synuclein toxicity
- PMID: 22319455
- PMCID: PMC3271059
- DOI: 10.1371/journal.pgen.1002488
The mitochondrial chaperone protein TRAP1 mitigates α-Synuclein toxicity
Abstract
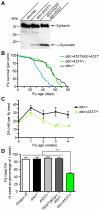
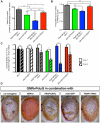
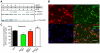
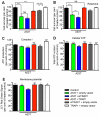
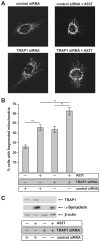
Overexpression or mutation of α-Synuclein is associated with protein aggregation and interferes with a number of cellular processes, including mitochondrial integrity and function. We used a whole-genome screen in the fruit fly Drosophila melanogaster to search for novel genetic modifiers of human [A53T]α-Synuclein-induced neurotoxicity. Decreased expression of the mitochondrial chaperone protein tumor necrosis factor receptor associated protein-1 (TRAP1) was found to enhance age-dependent loss of fly head dopamine (DA) and DA neuron number resulting from [A53T]α-Synuclein expression. In addition, decreased TRAP1 expression in [A53T]α-Synuclein-expressing flies resulted in enhanced loss of climbing ability and sensitivity to oxidative stress. Overexpression of human TRAP1 was able to rescue these phenotypes. Similarly, human TRAP1 overexpression in rat primary cortical neurons rescued [A53T]α-Synuclein-induced sensitivity to rotenone treatment. In human (non)neuronal cell lines, small interfering RNA directed against TRAP1 enhanced [A53T]α-Synuclein-induced sensitivity to oxidative stress treatment. [A53T]α-Synuclein directly interfered with mitochondrial function, as its expression reduced Complex I activity in HEK293 cells. These effects were blocked by TRAP1 overexpression. Moreover, TRAP1 was able to prevent alteration in mitochondrial morphology caused by [A53T]α-Synuclein overexpression in human SH-SY5Y cells. These results indicate that [A53T]α-Synuclein toxicity is intimately connected to mitochondrial dysfunction and that toxicity reduction in fly and rat primary neurons and human cell lines can be achieved using overexpression of the mitochondrial chaperone TRAP1. Interestingly, TRAP1 has previously been shown to be phosphorylated by the serine/threonine kinase PINK1, thus providing a potential link of PINK1 via TRAP1 to α-Synuclein.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. - PubMed
-
- Marsden CD. Neuromelanin and Parkinson's disease. J Neural Transm Suppl. 1983;19:121–141. - PubMed
-
- Lee FJ, Liu F. Genetic factors involved in the pathogenesis of Parkinson's disease. Brain Res Rev. 2008;58:354–364. - PubMed
-
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, et al. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

