A Ca²⁺-dependent chloride current and Ca²⁺ influx via Ca(v)1.2 ion channels play major roles in P2Y receptor-mediated pulmonary vasoconstriction
- PMID: 22320222
- PMCID: PMC3417463
- DOI: 10.1111/j.1476-5381.2012.01892.x
A Ca²⁺-dependent chloride current and Ca²⁺ influx via Ca(v)1.2 ion channels play major roles in P2Y receptor-mediated pulmonary vasoconstriction
Abstract
Background and purpose: ATP, UTP and UDP act at smooth muscle P2X and P2Y receptors to constrict rat intrapulmonary arteries, but the underlying signalling pathways are poorly understood. Here, we determined the roles of the Ca²⁺ -dependent chloride ion current (I(Cl,Ca)), Ca(v)1.2 ion channels and Ca²⁺ influx.
Experimental approach: Isometric tension was recorded from endothelium-denuded rat intrapulmonary artery rings (i.d. 200-500 µm) mounted on a wire myograph.
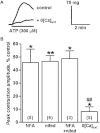
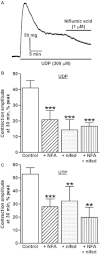
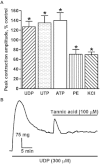
Key results: The I(Cl,Ca) blockers, niflumic acid and 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid and the Ca(v)1.2 channel blocker, nifedipine, reduced peak amplitude of contractions evoked by UTP and UDP by ∼45-50% and in a non-additive manner. Ca²⁺-free buffer inhibited responses by ∼70%. Niflumic acid and nifedipine similarly depressed contractions to ATP, but Ca²⁺-free buffer almost abolished the response. After peaking, contractions to UTP and UDP decayed slowly by 50-70% to a sustained plateau, which was rapidly inhibited by niflumic acid and nifedipine. Contractions to ATP, however, reversed rapidly and fully. Tannic acid contracted tissues per se and potentiated nucleotide-evoked contractions.
Conclusions and implications: I (Cl,Ca) and Ca²⁺ influx via Ca(v)1.2 ion channels contribute substantially and equally to contractions of rat intrapulmonary arteries evoked by UTP and UDP, via P2Y receptors. ATP also activates these mechanisms via P2Y receptors, but the greater dependence on extracellular Ca²⁺ most likely reflects additional influx through the P2X1 receptor pore. The lack of a sustained response to ATP is probably due to it acting at P2 receptor subtypes that desensitize rapidly. Thus multiple signalling mechanisms contribute to pulmonary artery vasoconstriction mediated by P2 receptors.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures



Similar articles
-
Purinergic regulation of pulmonary vascular tone.Purinergic Signal. 2024 Dec;20(6):595-606. doi: 10.1007/s11302-024-10010-5. Epub 2024 May 7. Purinergic Signal. 2024. PMID: 38713328 Free PMC article. Review.
-
Multiple P2Y receptors couple to calcium-dependent, chloride channels in smooth muscle cells of the rat pulmonary artery.Respir Res. 2005 Oct 26;6(1):124. doi: 10.1186/1465-9921-6-124. Respir Res. 2005. PMID: 16250909 Free PMC article.
-
Comparison of signalling mechanisms underlying UTP-evoked vasoconstriction of rat pulmonary and tail arteries.Eur J Pharmacol. 2018 Oct 15;837:45-52. doi: 10.1016/j.ejphar.2018.08.031. Epub 2018 Aug 29. Eur J Pharmacol. 2018. PMID: 30170065
-
Identification of contractile P2Y1, P2Y6, and P2Y12 receptors in rat intrapulmonary artery using selective ligands.J Pharmacol Exp Ther. 2012 Dec;343(3):755-62. doi: 10.1124/jpet.112.198051. Epub 2012 Sep 18. J Pharmacol Exp Ther. 2012. PMID: 22991416
-
How selective antagonists and genetic modification have helped characterise the expression and functions of vascular P2Y receptors.Purinergic Signal. 2025 Feb;21(1):11-22. doi: 10.1007/s11302-024-10016-z. Epub 2024 May 13. Purinergic Signal. 2025. PMID: 38740733 Free PMC article. Review.
Cited by
-
Antenatal hypoxia and pulmonary vascular function and remodeling.Curr Vasc Pharmacol. 2013 Sep;11(5):616-40. doi: 10.2174/1570161111311050006. Curr Vasc Pharmacol. 2013. PMID: 24063380 Free PMC article. Review.
-
Differentiation of human bronchial epithelial cells: role of hydrocortisone in development of ion transport pathways involved in mucociliary clearance.Am J Physiol Cell Physiol. 2016 Aug 1;311(2):C225-36. doi: 10.1152/ajpcell.00073.2016. Epub 2016 Jun 15. Am J Physiol Cell Physiol. 2016. PMID: 27306366 Free PMC article.
-
Purinergic regulation of pulmonary vascular tone.Purinergic Signal. 2024 Dec;20(6):595-606. doi: 10.1007/s11302-024-10010-5. Epub 2024 May 7. Purinergic Signal. 2024. PMID: 38713328 Free PMC article. Review.
References
-
- Baek EB, Yoo HY, Park SJ, Kim HS, Kim SD, Earm YE, et al. Luminal ATP-induced contraction of rabbit pulmonary arteries and role of purinoceptors in the regulation of pulmonary arterial pressure. Pflugers Arch. 2008;457:281–291. - PubMed
-
- Bakhramov A, Hartley SA, Salter KJ, Kozlowski RZ. Contractile agonists preferentially activate Cl− over K+ currents in arterial myocytes. Biochem Biophys Res Commun. 1996;1227:168–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

