Factors affecting the turnaround time for manufacturing, testing, and release of cellular therapy products prepared at multiple sites in support of multicenter cardiovascular regenerative medicine protocols: a Cardiovascular Cell Therapy Research Network (CCTRN) study
- PMID: 22320233
- PMCID: PMC3355200
- DOI: 10.1111/j.1537-2995.2011.03543.x
Factors affecting the turnaround time for manufacturing, testing, and release of cellular therapy products prepared at multiple sites in support of multicenter cardiovascular regenerative medicine protocols: a Cardiovascular Cell Therapy Research Network (CCTRN) study
Abstract
Background: Cellular therapy studies are often conducted at multiple clinical sites to accrue larger patient numbers. In many cases this necessitates use of localized good manufacturing practices facilities to supply the cells. To assure consistent quality, oversight by a quality assurance group is advisable. In this study we report the findings of such a group established as part of the Cardiovascular Cell Therapy Research Network (CCTRN) studies involving use of autologous bone marrow mononuclear cells (ABMMCs) to treat myocardial infarction and heart failure.
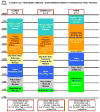
Study design and methods: Factors affecting cell manufacturing time were studied in 269 patients enrolled on three CCTRN protocols using automated cell processing system (Sepax, Biosafe SA)-separated ABMMCs. The cells were prepared at five good manufacturing practices cell processing facilities and delivered to local treatment sites or more distant satellite centers.
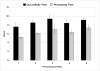
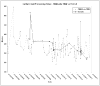
Results: Although the Sepax procedure takes only 90 minutes, the total time for processing was approximately 7 hours. Contributing to this were incoming testing and device preparation, release testing, patient randomization, and product delivery. The mean out-of-body time (OBT), which was to be less than 12 hours, averaged 9 hours. A detailed analysis of practices at each center revealed a variety of factors that contributed to this OBT.
Conclusion: We conclude that rapid cell enrichment procedures may give a false impression of the time actually required to prepare a cellular therapy product for release and administration. Institutional procedures also differ and can contribute to delays; however, in aggregate it is possible to achieve an overall manufacturing and testing time that is similar at multiple facilities.
© 2012 American Association of Blood Banks.
Figures

References
-
- Traverse JH, Henry TD, Vaughan DE, et al. Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–63. - PMC - PubMed
-
- Traverse JH, Henry TD, Vaughan DE, et al. Late TIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37:412–20. - PMC - PubMed
-
- Willerson JT, Perin EC, Ellis SG, et al. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (First Mononuclear Cells injected in the US [FOCUS]): Rationale and design. Am Heart J. 2010;160:215–23. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

