Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells
- PMID: 22320235
- PMCID: PMC7659194
- DOI: 10.1111/j.1349-7006.2012.02209.x
Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells
Abstract
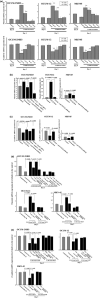
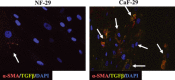
Tumor progression has been recognized as the product of evolving crosstalk between cancer cells and the surrounding stromal cells. Cancer-associated orthotopic myofibroblasts may be linked to the progression of gastric carcinomas. To understand the significance of orthotopic myofibroblasts, we examined the effects of cancer-associated orthotopic myofibroblasts on the malignant phenotype of gastric cancer cells. Three human gastric cancer cell lines (OCUM-2MD3, OCUM-12, MKN-45) and four human gastric fibroblast cell lines (cancer-associated orthotopic fibroblast [CaF]-29, CaF-33, normal orthotopic fibroblast [NF]-29, NF-33) were used. The cancer-associated orthotopic fibroblast cell lines CaF-29 and CaF-33 were established from a tumoral gastric wall, and normal orthotopic fibroblast NF-29 and NF-33 were established from a non-tumoral gastric wall. Fibroblasts that were α-smooth muscle actin-positive were defined as myofibroblasts. We examined the effects of cancer-associated orthotopic myofibroblasts on the aggressiveness of gastric cancer cells by wound-healing assay, invasion assay, and RT-PCR. The ratios of myofibroblasts in CaF-29 (33%) and CaF-33 (46%) were significantly (P < 0.001) greater than those in NF-29 (11%) or NF-33 (13%). Although all four orthotopic fibroblast lines increased the motility of gastric cancer cells, including migration and invasion ability, the motility-stimulating activity of cancer-associated fibroblasts (CaF-29 and CaF-33) was significantly higher than that of normal fibroblasts (NF-29 and NF-33). These motility-stimulating activities of cancer-associated orthotopic fibroblasts were downregulated by Smad2 siRNA treatment and anti-transforming growth factor-β neutralizing antibody. These findings suggest that cancer-associated orthotopic myofibroblasts may play an important role in the progression of gastric cancers and that transforming growth factor-β produced by myofibroblasts may be one of the factors associated with the aggressiveness of gastric carcinoma cells.
© 2012 Japanese Cancer Association.
Figures


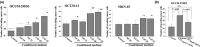

Similar articles
-
Upregulation of cancer-associated myofibroblasts by TGF-β from scirrhous gastric carcinoma cells.Br J Cancer. 2011 Sep 27;105(7):996-1001. doi: 10.1038/bjc.2011.330. Epub 2011 Aug 23. Br J Cancer. 2011. PMID: 21863023 Free PMC article.
-
Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-β signaling.Int J Cancer. 2014 Apr 15;134(8):1785-95. doi: 10.1002/ijc.28520. Epub 2013 Oct 24. Int J Cancer. 2014. PMID: 24155219
-
Activation of Transforming Growth Factor Beta 1 Signaling in Gastric Cancer-associated Fibroblasts Increases Their Motility, via Expression of Rhomboid 5 Homolog 2, and Ability to Induce Invasiveness of Gastric Cancer Cells.Gastroenterology. 2017 Jul;153(1):191-204.e16. doi: 10.1053/j.gastro.2017.03.046. Epub 2017 Apr 5. Gastroenterology. 2017. PMID: 28390866
-
Activated Fibroblast Program Orchestrates Tumor Initiation and Progression; Molecular Mechanisms and the Associated Therapeutic Strategies.Int J Mol Sci. 2019 May 7;20(9):2256. doi: 10.3390/ijms20092256. Int J Mol Sci. 2019. PMID: 31067787 Free PMC article. Review.
-
Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers.Cells. 2020 Sep 3;9(9):2027. doi: 10.3390/cells9092027. Cells. 2020. PMID: 32899119 Free PMC article. Review.
Cited by
-
Importance of human peritoneal mesothelial cells in the progression, fibrosis, and control of gastric cancer: inhibition of growth and fibrosis by tranilast.Gastric Cancer. 2018 Jan;21(1):55-67. doi: 10.1007/s10120-017-0726-5. Epub 2017 May 24. Gastric Cancer. 2018. PMID: 28540637 Free PMC article.
-
Bone marrow-derived stromal cells are associated with gastric cancer progression.Br J Cancer. 2015 Jul 28;113(3):443-52. doi: 10.1038/bjc.2015.236. Epub 2015 Jun 30. Br J Cancer. 2015. PMID: 26125445 Free PMC article.
-
Biological characteristics and genetic heterogeneity between carcinoma-associated fibroblasts and their paired normal fibroblasts in human breast cancer.PLoS One. 2013;8(4):e60321. doi: 10.1371/journal.pone.0060321. Epub 2013 Apr 5. PLoS One. 2013. PMID: 23577100 Free PMC article.
-
Reciprocal interaction between carcinoma-associated fibroblasts and squamous carcinoma cells through interleukin-1α induces cancer progression.Neoplasia. 2014 Nov 20;16(11):928-38. doi: 10.1016/j.neo.2014.09.003. eCollection 2014 Nov. Neoplasia. 2014. PMID: 25425967 Free PMC article.
-
Matrix Remodeling and Hyaluronan Production by Myofibroblasts and Cancer-Associated Fibroblasts in 3D Collagen Matrices.Gels. 2020 Sep 30;6(4):33. doi: 10.3390/gels6040033. Gels. 2020. PMID: 33008082 Free PMC article.
References
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. - PubMed
-
- Saito H, Tsujitani S, Oka S et al An elevated serum level of transforming growth factor‐beta 1 (TGF‐beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res 2000; 20: 4489–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

