From a marine neuropeptide to antimicrobial pseudopeptides containing aza-β(3)-amino acids: structure and activity
- PMID: 22320306
- PMCID: PMC3798980
- DOI: 10.1021/jm2011595
From a marine neuropeptide to antimicrobial pseudopeptides containing aza-β(3)-amino acids: structure and activity
Abstract
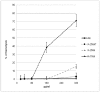
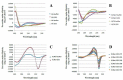
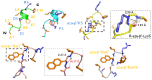
Incorporation of aza-β(3)-amino acids into an endogenous neuropeptide from mollusks (ALSGDAFLRF-NH(2)) with weak antimicrobial activity allows the design of new AMPs sequences. Depending on the nature of the substitution, this can render the pseudopeptides inactive or lead to a drastic enhancement of the antimicrobial activity without high cytotoxicity. Structural studies of the pseudopeptides carried out by NMR and circular dichroism show the impact of aza-β(3)-amino acids on peptide structure. The first three-dimensional structures of pseudopeptides containing aza-β(3)-amino acids in aqueous micellar SDS were determined and demonstrate that the hydrazino turn can be formed in aqueous solution. Thus, AMP activity can be modulated through structural modifications induced by the nature and the position of such amino acid analogues in the peptide sequences.
Figures





Similar articles
-
De novo cyclic pseudopeptides containing aza-β3-amino acids exhibiting antimicrobial activities.J Med Chem. 2012 Dec 27;55(24):10885-95. doi: 10.1021/jm3009037. Epub 2012 Dec 6. J Med Chem. 2012. PMID: 23148564
-
Selectivity Modulation and Structure of α/aza-β3 Cyclic Antimicrobial Peptides.Chemistry. 2018 Apr 20;24(23):6191-6201. doi: 10.1002/chem.201800152. Epub 2018 Mar 26. Chemistry. 2018. PMID: 29411917
-
Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties.Amino Acids. 2016 Feb;48(2):505-22. doi: 10.1007/s00726-015-2104-0. Epub 2015 Oct 8. Amino Acids. 2016. PMID: 26450121
-
Azapeptides as pharmacological agents.Curr Med Chem. 2005;12(5):589-97. doi: 10.2174/0929867310504050589. Curr Med Chem. 2005. PMID: 15777214 Review.
-
Helical Antimicrobial Peptide Foldamers Containing Non-proteinogenic Amino Acids.ChemMedChem. 2021 Apr 20;16(8):1226-1233. doi: 10.1002/cmdc.202000940. Epub 2021 Feb 10. ChemMedChem. 2021. PMID: 33565721 Review.
Cited by
-
Advances in Peptidomimetics for Next-Generation Therapeutics: Strategies, Modifications, and Applications.Chem Rev. 2025 Aug 13;125(15):7099-7166. doi: 10.1021/acs.chemrev.4c00989. Epub 2025 Jul 23. Chem Rev. 2025. PMID: 40698392 Free PMC article. Review.
-
OctoPartenopin: Identification and Preliminary Characterization of a Novel Antimicrobial Peptide from the Suckers of Octopus vulgaris.Mar Drugs. 2020 Jul 23;18(8):380. doi: 10.3390/md18080380. Mar Drugs. 2020. PMID: 32717885 Free PMC article.
-
Novel antibiotics effective against gram-positive and -negative multi-resistant bacteria with limited resistance.PLoS Biol. 2019 Jul 9;17(7):e3000337. doi: 10.1371/journal.pbio.3000337. eCollection 2019 Jul. PLoS Biol. 2019. PMID: 31287812 Free PMC article.
-
Consecutive hydrazino-Ugi-azide reactions: synthesis of acylhydrazines bearing 1,5-disubstituted tetrazoles.Beilstein J Org Chem. 2017 Dec 5;13:2596-2602. doi: 10.3762/bjoc.13.256. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 29259669 Free PMC article.
-
Synthesis of hybrid hydrazino peptides: protected vs unprotected chiral α-hydrazino acids.Springerplus. 2015 Sep 17;4:507. doi: 10.1186/s40064-015-1288-9. eCollection 2015. Springerplus. 2015. PMID: 26405627 Free PMC article.
References
-
- Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Biopolymers. 1998;47:415–433. - PubMed
-
- Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. - PubMed
-
- Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. - PubMed
-
- Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed

