Robust optimization of intensity modulated proton therapy
- PMID: 22320818
- PMCID: PMC3281975
- DOI: 10.1118/1.3679340
Robust optimization of intensity modulated proton therapy
Abstract
Purpose: Intensity modulated proton therapy (IMPT) is highly sensitive to range uncertainties and uncertainties caused by setup variation. The conventional inverse treatment planning of IMPT optimized based on the planning target volume (PTV) is not often sufficient to ensure robustness of treatment plans. In this paper, a method that takes the uncertainties into account during plan optimization is used to mitigate the influence of uncertainties in IMPT.
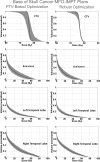
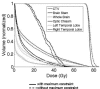
Methods: The authors use the so-called "worst-case robust optimization" to render IMPT plans robust in the face of uncertainties. For each iteration, nine different dose distributions are computed-one each for ± setup uncertainties along anteroposterior (A-P), lateral (R-L) and superior-inferior (S-I) directions, for ± range uncertainty, and the nominal dose distribution. The worst-case dose distribution is obtained by assigning the lowest dose among the nine doses to each voxel in the clinical target volume (CTV) and the highest dose to each voxel outside the CTV. Conceptually, the use of worst-case dose distribution is similar to the dose distribution achieved based on the use of PTV in traditional planning. The objective function value for a given iteration is computed using this worst-case dose distribution. The objective function used has been extended to further constrain the target dose inhomogeneity.
Results: The worst-case robust optimization method is applied to a lung case, a skull base case, and a prostate case. Compared with IMPT plans optimized using conventional methods based on the PTV, our method yields plans that are considerably less sensitive to range and setup uncertainties. An interesting finding of the work presented here is that, in addition to reducing sensitivity to uncertainties, robust optimization also leads to improved optimality of treatment plans compared to the PTV-based optimization. This is reflected in reduction in plan scores and in the lower normal tissue doses for the same coverage of the target volume when subjected to uncertainties.
Conclusions: The authors find that the worst-case robust optimization provides robust target coverage without sacrificing, and possibly even improving, the sparing of normal tissues. Our results demonstrate the importance of robust optimization. The authors assert that all IMPT plans should be robustly optimized.
Figures




References
-
- Register S. P., Zhang X., Mohan R., and Chang J. Y., “Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer,” Int. J. Radiat. Oncol., Biol., Phys. 80, 1015–1022 (2010). 10.1016/j.ijrobp.2010.03.012 - DOI - PMC - PubMed
-
- Zhang X. D., Li Y. P., Pan X. N., Li X. Q., Mohan R., Komaki R., Cox J. D., and Chang J. Y., “Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical raditherapy for extensive stage IIIB non-small-cell lung cancer: A virtual clinical study,” Int. J. Radiat. Oncol., Biol., Phys. 77, 357–366 (2010). 10.1016/j.ijrobp.2009.04.028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

