A new PICTure of nucleolar stress
- PMID: 22320853
- PMCID: PMC7659309
- DOI: 10.1111/j.1349-7006.2012.02219.x
A new PICTure of nucleolar stress
Abstract
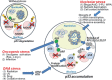
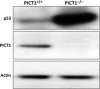
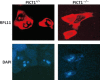
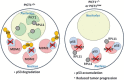
Cell growth demands new protein synthesis, which requires nucleolar ribosomal functions. Ribosome biogenesis consumes a large proportion of the cell's resources and energy, and so is tightly regulated through an intricate signaling network to guarantee fidelity. Thus, events that impair ribosome biogenesis cause nucleolar stress. In response to this stress, several nucleolar ribosomal proteins (RPs) translocate to the nucleoplasm and bind to MDM2. MDM2-mediated ubiquitination and degradation of the tumor suppressor p53 is then blocked, resulting in p53 accumulation and the induction of p53-dependent cell cycle arrest and apoptosis. Nucleolar stress is therefore a quality control surveillance mechanism that monitors the synthesis and assembly of the rRNA and protein components of ribosomes. Although nucleolar stress signaling pathways have been extensively analyzed, critical questions remain about their regulatory mechanisms. For example, how do RPs translocate from the nucleolus to the nucleoplasm to exert their functions, and do these p53-regulating RPs influence the prognosis of human cancer patients? Our laboratory recently identified the nucleolar protein PICT1 as a novel regulator of nucleolar stress. PICT1 sequesters the ribosomal protein RPL11 in the nucleolus, preventing it from binding to MDM2. MDM2 is then free to degrade p53, favoring tumor cell growth. Accordingly, the level of PICT1 in a tumor is becoming a useful prognostic marker for human cancers. This review summarizes the evidence that links nucleolar stress to tumorigenesis, and casts PICT1 as an oncogenic player in human cancer biology.
© 2012 Japanese Cancer Association.
Figures
References
-
- Vousden KH, Ryan KM. p53 and metabolism. Nat Rev 2009; 9: 691–700. - PubMed
-
- Suzuki HI, Yamagata K, Sugimoto K et al Modulation of microRNA processing by p53. Nature 2009; 460: 529–33. - PubMed
-
- Haupt Y, Maya R, Kazaz A et al Mdm2 promotes the rapid degradation of p53. Nature 1997; 387: 296–9. - PubMed
-
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2‐deficient mice by deletion of p53. Nature 1995; 378: 203–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

