Alcohol, insulin resistance and the liver-brain axis
- PMID: 22320914
- PMCID: PMC4550293
- DOI: 10.1111/j.1440-1746.2011.07023.x
Alcohol, insulin resistance and the liver-brain axis
Abstract
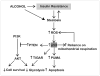
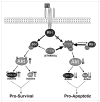
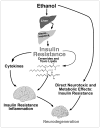
Chronic alcohol exposure inhibits insulin and insulin-like growth factor signaling in the liver and brain by impairing the signaling cascade at multiple levels. These alterations produced by alcohol cause severe hepatic and central nervous system insulin resistance as the cells fail to adequately transmit signals downstream through Erk/mitogen-activated protein kinase (MAPK), which is needed for DNA synthesis and liver regeneration, and phosphatidylinositol 3-kinase (PI3K), which promotes growth, survival, cell motility, glucose utilization, plasticity, and energy metabolism. The robust inhibition of insulin signaling in liver and brain is augmented by additional factors involving the activation of phosphatases such as phosphatase and tensin homologue (PTEN), which further impairs insulin signaling through PI3K/Akt. Thus, intact insulin signaling is important for neuronal survival. Chronic alcohol consumption produces steatohepatitis, which also promotes hepatic insulin resistance, oxidative stress and injury, with the attendant increased generation of "toxic lipids" such as ceramides that increase insulin resistance. The PI3K/Akt signaling cascade is altered by direct interaction with ceramides as well as through PTEN upregulation as a downstream target gene of enhanced p53 transcriptional activity. Cytotoxic ceramides transferred from the liver to the blood can enter the brain due to their lipid-soluble nature, and thereby exert neurodegenerative effects via a liver-brain axis. We postulate that the neurotoxic and neurodegenerative effects of liver-derived ceramides activate pro-inflammatory cytokines and increase lipid adducts and insulin resistance in the brain to impair cognitive and motor function. These observations are discussed in the context of insulin sensitizers as potential cytoprotective agents against liver and brain injury induced by alcohol.
© 2012 Journal of Gastroenterology and Hepatology Foundation and Blackwell Publishing Asia Pty Ltd.
Conflict of interest statement
Conflicts of interest: the authors have no conflict of interest.
Figures
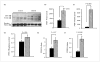
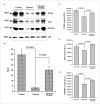
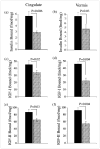
 ) BrdU(+)/total hepatocytes; (▨) PCNA; (▩) AAH; (▥) GAPDH.
) BrdU(+)/total hepatocytes; (▨) PCNA; (▩) AAH; (▥) GAPDH.
References
-
- Ullrich A, Bell JR, Chen EY, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–61. - PubMed
-
- Bonini JA, Colca JR, Dailey C, White M, Hofmann C. Compensatory alterations for insulin signal transduction and glucose transport in insulin-resistant diabetes. Am J Physiol. 1995;269 (4 Pt 1):E759–65. - PubMed
-
- Skolnik EY, Batzer A, Li N, et al. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–5. - PubMed
-
- Tanaka S, Ito T, Wands JR. Neoplastic transformation induced by insulin receptor substrate-1 overexpression requires an interaction with both Grb2 and Syp signaling molecules. J Biol Chem. 1996;271:14610–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

