Preventing flow-metabolism uncoupling acutely reduces axonal injury after traumatic brain injury
- PMID: 22321027
- PMCID: PMC3335110
- DOI: 10.1089/neu.2011.2161
Preventing flow-metabolism uncoupling acutely reduces axonal injury after traumatic brain injury
Abstract
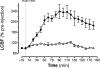
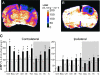
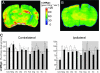
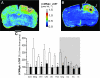
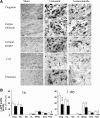
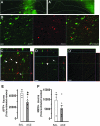
We have previously presented evidence that the development of secondary traumatic axonal injury is related to the degree of local cerebral blood flow (LCBF) and flow-metabolism uncoupling. We have now tested the hypothesis that augmenting LCBF in the acute stages after brain injury prevents further axonal injury. Data were acquired from rats with or without acetazolamide (ACZ) that was administered immediately following controlled cortical impact injury to increase cortical LCBF. Local cerebral metabolic rate for glucose (LCMRglc) and LCBF measurements were obtained 3 h post-trauma in the same rat via ¹⁸F-fluorodeoxyglucose and ¹⁴C-iodoantipyrine co-registered autoradiographic images, and compared to the density of damaged axonal profiles in adjacent sections, and in additional groups at 24 h used to assess different populations of injured axons stereologically. ACZ treatment significantly and globally elevated LCBF twofold above untreated-injured rats at 3 h (p<0.05), but did not significantly affect LCMRglc. As a result, ipsilateral LCMRglc:LCBF ratios were reduced by twofold to sham-control levels, and the density of β-APP-stained axons at 24 h was significantly reduced in most brain regions compared to the untreated-injured group (p<0.01). Furthermore, early LCBF augmentation prevented the injury-associated increase in the number of stained axons from 3-24 h. Additional robust stereological analysis of impaired axonal transport and neurofilament compaction in the corpus callosum and cingulum underlying the injury core confirmed the amelioration of β-APP axon density, and showed a trend, but no significant effect, on RMO14-positive axons. These data underline the importance of maintaining flow-metabolism coupling immediately after injury in order to prevent further axonal injury, in at least one population of injured axons.
Figures

Similar articles
-
Relationship between flow-metabolism uncoupling and evolving axonal injury after experimental traumatic brain injury.J Cereb Blood Flow Metab. 2004 Sep;24(9):1025-36. doi: 10.1097/01.WCB.0000129415.34520.47. J Cereb Blood Flow Metab. 2004. PMID: 15356423
-
Diminution of metabolism/blood flow uncoupling following traumatic brain injury in rats in response to high-dose human albumin treatment.J Neurosurg. 2001 Mar;94(3):499-509. doi: 10.3171/jns.2001.94.3.0499. J Neurosurg. 2001. PMID: 11235957
-
Influence of early posttraumatic hypothermia therapy on local cerebral blood flow and glucose metabolism after fluid-percussion brain injury.J Neurosurg. 1999 Mar;90(3):510-9. doi: 10.3171/jns.1999.90.3.0510. J Neurosurg. 1999. PMID: 10067921
-
Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats.Am J Physiol. 1997 Jun;272(6 Pt 2):H2859-68. doi: 10.1152/ajpheart.1997.272.6.H2859. Am J Physiol. 1997. PMID: 9227566
-
Transient middle cerebral artery occlusion by intraluminal suture: I. Three-dimensional autoradiographic image-analysis of local cerebral glucose metabolism-blood flow interrelationships during ischemia and early recirculation.J Cereb Blood Flow Metab. 1997 Dec;17(12):1266-80. doi: 10.1097/00004647-199712000-00002. J Cereb Blood Flow Metab. 1997. PMID: 9397026
Cited by
-
Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage.PLoS One. 2015 Feb 6;10(2):e0118061. doi: 10.1371/journal.pone.0118061. eCollection 2015. PLoS One. 2015. PMID: 25659079 Free PMC article.
-
Blast Overpressure Waves Induce Transient Anxiety and Regional Changes in Cerebral Glucose Metabolism and Delayed Hyperarousal in Rats.Front Neurol. 2015 Jun 17;6:132. doi: 10.3389/fneur.2015.00132. eCollection 2015. Front Neurol. 2015. PMID: 26136722 Free PMC article.
-
FDG-PET imaging in mild traumatic brain injury: a critical review.Front Neuroenergetics. 2014 Jan 9;5:13. doi: 10.3389/fnene.2013.00013. Front Neuroenergetics. 2014. PMID: 24409143 Free PMC article. Review.
-
Bi-directional changes in fractional anisotropy after experiment TBI: Disorganization and reorganization?Neuroimage. 2016 Jun;133:129-143. doi: 10.1016/j.neuroimage.2016.03.012. Epub 2016 Mar 11. Neuroimage. 2016. PMID: 26975556 Free PMC article.
-
Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: An analysis of insurance claims data, 2004-2014.Epilepsy Res. 2018 Oct;146:41-49. doi: 10.1016/j.eplepsyres.2018.07.012. Epub 2018 Jul 23. Epilepsy Res. 2018. PMID: 30071385 Free PMC article.
References
-
- Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. - PubMed
-
- Belayev L. Alonso O.F. Huh P.W. Zhao W. Busto R. Ginsberg M.D. Post treatment with high-dose albumin reduces histopathological damage and improves neurological deficit following fluid percussion brain injury in rats. J. Neurotrauma. 1999;16:445–453. - PubMed
-
- Benjamini Y. Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. 1995;57:289–300.
-
- Bergsneider M. Hovda D.A. Shalmon E. Kelly D.F. Vespa P.M. Martin N.A. Phelps M.E. McArthur D.L. Caron M.J. Kraus J.F. Becker D.P. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. - PubMed
-
- Bickler P.E. Litt L. Banville D.L. Severinghaus J.W. Effects of acetazolamide on cerebral acid-base balance. J. Appl. Physiol. 1988;65:422–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

