A senescent cell bystander effect: senescence-induced senescence
- PMID: 22321662
- PMCID: PMC3488292
- DOI: 10.1111/j.1474-9726.2012.00795.x
A senescent cell bystander effect: senescence-induced senescence
Abstract
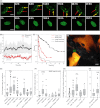
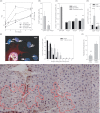
Senescent cells produce and secrete various bioactive molecules including interleukins, growth factors, matrix-degrading enzymes and reactive oxygen species (ROS). Thus, it has been proposed that senescent cells can damage their local environment, and a stimulatory effect on tumour cell growth and invasiveness has been documented. However, it was unknown what effect, if any, senescent cells have on their normal, proliferation-competent counterparts. We show here that senescent cells induce a DNA damage response, characteristic for senescence, in neighbouring cells via gap junction-mediated cell-cell contact and processes involving ROS. Continuous exposure to senescent cells induced cell senescence in intact bystander fibroblasts. Hepatocytes bearing senescence markers clustered together in mice livers. Thus, senescent cells can induce a bystander effect, spreading senescence towards their neighbours in vitro and, possibly, in vivo.
© 2012 The Authors. Aging Cell © 2012 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures
References
-
- Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. - PubMed
-
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. - PubMed
-
- Chen S, Zhao Y, Zhao G, Han W, Bao L, Yu KN, Wu L. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutat. Res. 2009;666:68–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F010966/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB ⁄ C008200 ⁄ 1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I020748/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0601333/MRC_/Medical Research Council/United Kingdom
- G0900686/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

