Ion and solute transport by Prestin in Drosophila and Anopheles
- PMID: 22321763
- PMCID: PMC3482613
- DOI: 10.1016/j.jinsphys.2012.01.009
Ion and solute transport by Prestin in Drosophila and Anopheles
Abstract
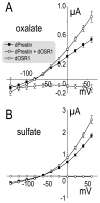
The gut and Malpighian tubules of insects are the primary sites of active solute and water transport for controlling hemolymph and urine composition, pH, and osmolarity. These processes depend on ATPase (pumps), channels and solute carriers (Slc proteins). Maturation of genomic databases enables us to identify the putative molecular players for these processes. Anion transporters of the Slc4 family, AE1 and NDAE1, have been reported as HCO(3)(-) transporters, but are only part of the story. Here we report Dipteran (Drosophila melanogaster (d) and Anopheles gambiae (Ag)) anion exchangers, belonging to the Slc26 family, which are multi-functional anion exchangers. One Drosophila and two Ag homologues of mammalian Slc26a5 (Prestin) and Slc26a6 (aka, PAT1, CFEX) were identified and designated dPrestin, AgPrestinA and AgPrestinB. dPrestin and AgPrestinB show electrogenic anion exchange (Cl(-)/nHCO(3)(-), Cl(-)/SO(4)(2-) and Cl(-)/oxalate(2-)) in an oocyte expression system. Since these transporters are the only Dipteran Slc26 proteins whose transport is similar to mammalian Slc26a6, we submit that Dipteran Prestin are functional and even molecular orthologues of mammalian Slc26a6. OSR1 kinase increases dPrestin ion transport, implying another set of physiological processes controlled by WNK/SPAK signaling in epithelia. All of these mRNAs are highly expressed in the gut and Malpighian tubules. Dipteran Prestin proteins appear suited for central roles in bicarbonate, sulfate and oxalate metabolism including generating the high pH conditions measured in the Dipteran midgut lumen. Finally, we present and discuss Drosophila genetic models that integrate these processes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization.Am J Physiol Cell Physiol. 2011 Aug;301(2):C289-303. doi: 10.1152/ajpcell.00089.2011. Epub 2011 May 18. Am J Physiol Cell Physiol. 2011. PMID: 21593449 Free PMC article.
-
Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (Slc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis.Am J Physiol Renal Physiol. 2016 Jan 15;310(2):F152-9. doi: 10.1152/ajprenal.00406.2015. Epub 2015 Nov 4. Am J Physiol Renal Physiol. 2016. PMID: 26538444 Free PMC article.
-
Expression, regulation and the role of SLC26 Cl-/HCO3- exchangers in kidney and gastrointestinal tract.Novartis Found Symp. 2006;273:91-102; discussion 103-6, 261-4. Novartis Found Symp. 2006. PMID: 17120763 Review.
-
Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity.J Biol Chem. 2005 Mar 4;280(9):8564-80. doi: 10.1074/jbc.M411703200. Epub 2004 Nov 17. J Biol Chem. 2005. PMID: 15548529
-
Molecular physiology of SLC4 anion exchangers.Exp Physiol. 2006 Jan;91(1):153-61. doi: 10.1113/expphysiol.2005.031765. Epub 2005 Oct 20. Exp Physiol. 2006. PMID: 16239253 Review.
Cited by
-
Physiology, Development, and Disease Modeling in the Drosophila Excretory System.Genetics. 2020 Feb;214(2):235-264. doi: 10.1534/genetics.119.302289. Genetics. 2020. PMID: 32029579 Free PMC article. Review.
-
Organ transcriptomes of the lucinid clam Loripes orbiculatus (Poli, 1791) provide insights into their specialised roles in the biology of a chemosymbiotic bivalve.BMC Genomics. 2019 Nov 7;20(1):820. doi: 10.1186/s12864-019-6177-0. BMC Genomics. 2019. PMID: 31699041 Free PMC article.
-
Genomic advances in the study of the mosquito vector during avian malaria infection.Parasitology. 2023 Dec;150(14):1330-1339. doi: 10.1017/S0031182023000756. Epub 2023 Aug 24. Parasitology. 2023. PMID: 37614176 Free PMC article. Review.
-
WNK Kinases in Development and Disease.Curr Top Dev Biol. 2017;123:1-47. doi: 10.1016/bs.ctdb.2016.08.004. Epub 2016 Sep 28. Curr Top Dev Biol. 2017. PMID: 28236964 Free PMC article. Review.
-
The Drosophila NKCC Ncc69 is required for normal renal tubule function.Am J Physiol Cell Physiol. 2012 Oct 15;303(8):C883-94. doi: 10.1152/ajpcell.00201.2012. Epub 2012 Aug 22. Am J Physiol Cell Physiol. 2012. PMID: 22914641 Free PMC article.
References
-
- Alper SL. Familial renal tubular acidosis. Journal of nephrology. 2010;23(Suppl 16):S57–76. - PubMed
-
- Boudko DY, Moroz LL, Linser PJ, Trimarchi JR, Smith PJ, Harvey WR. In situ analysis of pH gradients in mosquito larvae using non-invasive, self-referencing, pH-sensitive microelectrodes. J Exp Biol. 2001b;204:691–699. - PubMed
-
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

