Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice
- PMID: 22322300
- PMCID: PMC3354589
- DOI: 10.1016/j.ajpath.2011.12.036
Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice
Abstract
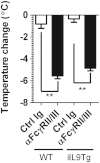
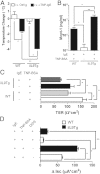
Food-triggered anaphylaxis can encompass a variety of symptoms that affect multiple organ systems and can be life threatening. The molecular distinction between non-life-threatening and life-threatening modes of such anaphylaxis has not yet been delineated. In this study, we sought to identify the specific immune functions that regulate the severity of oral antigen-induced anaphylaxis. We thus developed an experimental mouse model in which repeated oral challenge of ovalbumin-primed mice induced an FcεRI- and IgE-dependent oral antigen-triggered anaphylaxis that involved multiple organ systems. Strikingly, the severity of the systemic symptoms of anaphylaxis (eg, hypothermia) positively correlated with the levels of intestinal mast cells (r = -0.53; P < 0.009). In addition, transgenic mice with both increased intestinal and normal systemic levels of mast cells showed increased severity of both intestinal and extra-intestinal symptoms of IgE-mediated passive as well as oral antigen- and IgE-triggered anaphylaxis. In conclusion, these observations indicate that the density of intestinal mast cells controls the severity of oral antigen-induced anaphylaxis. Thus, an awareness of intestinal mast cell levels in patients with food allergies may aid in determining their susceptibility to life-threatening anaphylaxis and may eventually aid in the treatment of food-triggered anaphylaxis.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



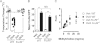
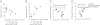


References
-
- Sampson H.A. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–1608. - PubMed
-
- Ross M.P., Ferguson M., Street D., Klontz K., Schroeder T., Luccioli S. Analysis of food-allergic and anaphylactic events in the national electronic injury surveillance system. J Allergy Clin Immunol. 2008;121:166–171. - PubMed
-
- De Smit V., Cameron P.A., Rainer T.H. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–388. - PubMed
-
- Brown A.F., McKinnon D., Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–866. - PubMed
-
- Simons E.R., Chad Z.H., Gold M. Anaphylaxis in children: realtime reporting from a national network. Allergy Clin Immunol Int J World Allergy Org. 2004;(Supplement 1):242.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

