Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-κB signaling pathways
- PMID: 22322442
- PMCID: PMC3522120
- DOI: 10.1002/ijc.27478
Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-κB signaling pathways
Abstract
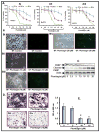
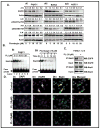
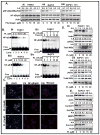
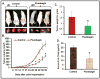
Pancreatic cancer (PC) is the most aggressive malignant disease, ranks as the fourth most leading cause of cancer-related death among men and women in the United States. We present here that plumbagin (PL), a quinoid constituent isolated from the roots of the medicinal plant Plumbago zeylanica L, inhibits the growth of PC cells both in vitro and in vivo model systems. PL treatment induces apoptosis and inhibits cell viability of PC cells (PANC1, BxPC3 and ASPC1). In addition, i.p. administration of PL (2 mg/kg body weight, 5 days a week) in severe combined immunodeficiency (SCID) mice beginning 3 days after ectopic implantation of PANC1 cells resulted in a significant (P < 0.01) inhibition of both tumor weight and volume. PL treatment inhibited (1) constitutive expression of epidermal growth factor receptor (EGFR), pStat3Tyr705 and pStat3Ser727, (2) DNA binding of Stat3 and (3) physical interaction of EGFR with Stat3, in both cultured PANC1 cells and their xenograft tumors. PL treatment also inhibited phosphorylation and DNA-binding activity of NF-κB in both cultured PC cells (PANC1 and ASPC1) and in PANC1 cells xenograft tumors. Downstream target genes (cyclin D1, MMP9 and Survivin) of Stat3 and NF-κB were similarly inhibited. These results suggest that PL may be used as a novel therapeutic agent against human PC. Published 2012 Wiley-Liss, Inc. This article is a US Government work, and, as such, is in the public domain in the United States of America.
Copyright © 2012 UICC.
Figures

Similar articles
-
α-Mangostin: a dietary antioxidant derived from the pericarp of Garcinia mangostana L. inhibits pancreatic tumor growth in xenograft mouse model.Antioxid Redox Signal. 2014 Aug 10;21(5):682-99. doi: 10.1089/ars.2013.5212. Epub 2014 Feb 6. Antioxid Redox Signal. 2014. PMID: 24295217 Free PMC article.
-
Plumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3M-luciferase cells in an orthotopic xenograft mouse model.Mol Oncol. 2013 Jun;7(3):428-39. doi: 10.1016/j.molonc.2012.12.001. Epub 2012 Dec 14. Mol Oncol. 2013. PMID: 23273564 Free PMC article.
-
Combined targeting of STAT3/NF-κB/COX-2/EP4 for effective management of pancreatic cancer.Clin Cancer Res. 2014 Mar 1;20(5):1259-73. doi: 10.1158/1078-0432.CCR-13-1664. Epub 2014 Feb 11. Clin Cancer Res. 2014. PMID: 24520096 Free PMC article.
-
Concurrent inhibition of NF-kappaB, cyclooxygenase-2, and epidermal growth factor receptor leads to greater anti-tumor activity in pancreatic cancer.J Cell Biochem. 2010 May;110(1):171-81. doi: 10.1002/jcb.22523. J Cell Biochem. 2010. Retraction in: J Cell Biochem. 2016 Aug;117(8):1961. doi: 10.1002/jcb.25586. PMID: 20213764 Free PMC article. Retracted.
-
Pancreatic cancer: pathogenesis, prevention and treatment.Toxicol Appl Pharmacol. 2007 Nov 1;224(3):326-36. doi: 10.1016/j.taap.2006.11.007. Epub 2006 Nov 11. Toxicol Appl Pharmacol. 2007. PMID: 17174370 Free PMC article. Review.
Cited by
-
Plumbagin induces RPE cell cycle arrest and apoptosis via p38 MARK and PI3K/AKT/mTOR signaling pathways in PVR.BMC Complement Altern Med. 2018 Mar 13;18(1):89. doi: 10.1186/s12906-018-2155-3. BMC Complement Altern Med. 2018. PMID: 29534723 Free PMC article.
-
Challenges and advances in mouse modeling for human pancreatic tumorigenesis and metastasis.Cancer Metastasis Rev. 2013 Jun;32(1-2):83-107. doi: 10.1007/s10555-012-9408-2. Cancer Metastasis Rev. 2013. PMID: 23114842 Free PMC article. Review.
-
Plumbagin Suppresses the Invasion of HER2-Overexpressing Breast Cancer Cells through Inhibition of IKKα-Mediated NF-κB Activation.PLoS One. 2016 Oct 11;11(10):e0164064. doi: 10.1371/journal.pone.0164064. eCollection 2016. PLoS One. 2016. Retraction in: PLoS One. 2019 Jan 18;14(1):e0207273. doi: 10.1371/journal.pone.0207273. PMID: 27727280 Free PMC article. Retracted.
-
Inhibitory Effects of Plumbagin on Retinal Pigment Epithelial Cell Epithelial-Mesenchymal Transition In Vitro and In Vivo.Med Sci Monit. 2018 Mar 13;24:1502-1510. doi: 10.12659/msm.906265. Med Sci Monit. 2018. PMID: 29532788 Free PMC article.
-
The attenuating effects of plumbagin on pro-inflammatory cytokine expression in LPS-activated BV-2 microglial cells.J Neuroimmunol. 2017 Dec 15;313:129-137. doi: 10.1016/j.jneuroim.2017.09.007. Epub 2017 Sep 20. J Neuroimmunol. 2017. PMID: 28950995 Free PMC article.
References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. - PubMed
-
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69. - PubMed
-
- Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol Rep. 2007;18(1):151–5. - PubMed
-
- Tsiambas E, Karameris A, Lazaris AC, Talieri M, Triantafillidis JK, Cheracakis P, Manaios L, Gerontopoulos K, Patsouris E, Lygidakis NJ. EGFR alterations in pancreatic ductal adenocarcinoma: a chromogenic in situ hybridization analysis based on tissue microarrays. Hepatogastroenterology. 2006;53 (69):452–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

