The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation
- PMID: 22323289
- PMCID: PMC3315811
- DOI: 10.1091/mbc.E11-09-0772
The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation
Abstract
Members of the bacterial and mitochondrial iron-sulfur cluster (ISC) assembly machinery include the so-called A-type ISC proteins, which support the assembly of a subset of Fe/S apoproteins. The human genome encodes two A-type proteins, termed ISCA1 and ISCA2, which are related to Saccharomyces cerevisiae Isa1 and Isa2, respectively. An additional protein, Iba57, physically interacts with Isa1 and Isa2 in yeast. To test the cellular role of human ISCA1, ISCA2, and IBA57, HeLa cells were depleted for any of these proteins by RNA interference technology. Depleted cells contained massively swollen and enlarged mitochondria that were virtually devoid of cristae membranes, demonstrating the importance of these proteins for mitochondrial biogenesis. The activities of mitochondrial [4Fe-4S] proteins, including aconitase, respiratory complex I, and lipoic acid synthase, were diminished following depletion of the three proteins. In contrast, the mitochondrial [2Fe-2S] enzyme ferrochelatase and cellular heme content were unaffected. We further provide evidence against a localization and direct Fe/S protein maturation function of ISCA1 and ISCA2 in the cytosol. Taken together, our data suggest that ISCA1, ISCA2, and IBA57 are specifically involved in the maturation of mitochondrial [4Fe-4S] proteins functioning late in the ISC assembly pathway.
Figures
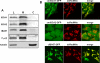

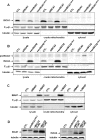



References
-
- Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A) Hum Mol Genet. 1999;8:743–749. - PubMed
-
- Brazzolotto X, Gaillard J, Pantopoulos K, Hentze MW, Moulis JM. Human cytoplasmic aconitase (iron regulatory protein 1) is converted into its [3Fe-4S] form by hydrogen peroxide in vitro but is not activated for iron-responsive element binding. J Biol Chem. 1999;274:21625–21630. - PubMed
-
- Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, Levi S, Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110:1353–1358. - PubMed
-
- Cameron JM, Janer A, Levandovskiy V, Mackay N, Rouault TA, Tong WH, Ogilvie I, Shoubridge EA, Robinson BH. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am J Hum Genet. 2011;89:486–495. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

