Analysis of NLRP3 in the development of allergic airway disease in mice
- PMID: 22323538
- PMCID: PMC3294123
- DOI: 10.4049/jimmunol.1102488
Analysis of NLRP3 in the development of allergic airway disease in mice
Abstract
The contribution of NLRP3, a member of the nucleotide-binding domain leucine-rich repeat-containing (NLR) family, to the development of allergic airway disease is currently controversial. In this study, we used multiple allergic asthma models to examine the physiologic role of NLRP3. We found no significant differences in airway eosinophilia, histopathologic condition, mucus production, and airway hyperresponsiveness between wild-type and Nlrp3(-/-) mice in either acute (alum-dependent) or chronic (alum-independent) OVA models. In addition to the OVA model, we did not detect a role for NLRP3 in the development of allergic airway disease induced by either acute or chronic house dust mite Ag exposure. Although we did not observe significant phenotypic differences in any of the models tested, we did note a significant reduction of IL-13 and IL-33 in Nlrp3(-/-) mice compared with wild-type controls in the chronic OVA model without added alum. In all of the allergic airway disease models, the NLRP3 inflammasome-associated cytokines IL-1β and IL-18 in the lung were below the level of detection. In sum, this report surveyed four different allergic asthma models and found a modest and selected role for NLRP3 in the alum-free OVA model. However, this difference did not greatly alter the clinical outcome of the disease. This finding suggests that the role of NLRP3 in allergic asthma must be re-evaluated.
Figures



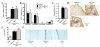

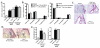
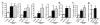


References
-
- Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy. 2009;39:12–9. - PubMed
-
- Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–77. - PubMed
-
- Simpson A, Martinez FD. The role of lipopolysaccharide in the development of atopy in humans. Clin Exp Allergy. 2010;40:209–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- A1063031/PHS HHS/United States
- F32 AI082895/AI/NIAID NIH HHS/United States
- F32-AI-082895-01/AI/NIAID NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- DK38108/DK/NIDDK NIH HHS/United States
- K01 DK092355/DK/NIDDK NIH HHS/United States
- R01 HL068141/HL/NHLBI NIH HHS/United States
- DE016326/DE/NIDCR NIH HHS/United States
- R01 DE016326/DE/NIDCR NIH HHS/United States
- T32 HL083828/HL/NHLBI NIH HHS/United States
- T32-CA009156/CA/NCI NIH HHS/United States
- U19-AI077437/AI/NIAID NIH HHS/United States
- T32-AR007416/AR/NIAMS NIH HHS/United States
- HL06814/HL/NHLBI NIH HHS/United States
- U19 AI067798/AI/NIAID NIH HHS/United States
- T32 AR007416/AR/NIAMS NIH HHS/United States
- U19 AI077437/AI/NIAID NIH HHS/United States
- U19-AI067798/AI/NIAID NIH HHS/United States
- P01 DK038108/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

