Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial
- PMID: 22323576
- PMCID: PMC3432241
- DOI: 10.1183/09031936.00155811
Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial
Abstract
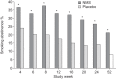
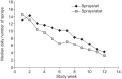
A nicotine mouth spray has advantages over other acute forms of nicotine replacement therapy, such as a faster uptake of nicotine and faster relief of craving. This multicentre, randomised (2:1), double-blind, placebo-controlled efficacy and safety study evaluated self-reported, carbon monoxide-verified continuous abstinence from smoking from week 2 until weeks 6, 24, and 52 in 479 smokers (≥ 1 cigarette per day) who were treated with either active (n=318) or placebo (n=161) spray for 12 weeks and low-intensity counselling at three smoking cessation clinics in Denmark and Germany. Active treatment yielded significantly higher continuous abstinence rates than placebo from week 2 until week 6 (26.1% versus 16.1%; relative success rate (RR) 1.62, 95% CI 1.09-2.41), week 24 (15.7% versus 6.8%; RR 2.30, 95% CI 1.23-4.30), and week 52 (13.8% versus 5.6%; RR 2.48, 95% CI 1.24-4.94). Most adverse events were mild to moderate, and 9.1% of subjects on active spray withdrew due to adverse events, compared to 7.5% on placebo. The overall rate of treatment-related adverse events was 87.4% with active spray versus 71.4% with placebo spray. Nicotine mouth spray delivered significantly higher 6-, 24- and 52-week continuous abstinence rates than placebo.
Trial registration: ClinicalTrials.gov NCT00759304 NCT00766584.
Conflict of interest statement
Statements of interest for all authors of this manuscript and for the study itself can be found at
Figures
References
-
- Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008; 1: CD000146. - PubMed
-
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 2008; 83: 531–541 - PubMed
-
- Kraiczi H, Hansson A, Perfekt R. Single-dose pharmacokinetics of nicotine when given with a novel mouth spray for nicotine replacement therapy. Nicotine Tob Res 2011; 13: 1176–1182 - PubMed
-
- American PsychiatricAssociation(APA) Diagnostic and Statistical Manual of Mental Disorders 4th Edn Text Revision (DSM–IV-TR) Washington, APA, 2000
-
- Tønnesen P, Paoletti P, Gustavsson G, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Eur Respir J 1999; 13: 238–246 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
