Attentional selection of location and modality in vision and touch modulates low-frequency activity in associated sensory cortices
- PMID: 22323628
- PMCID: PMC3362245
- DOI: 10.1152/jn.00973.2011
Attentional selection of location and modality in vision and touch modulates low-frequency activity in associated sensory cortices
Abstract
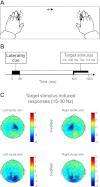
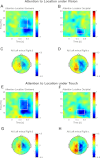
Selective attention allows us to focus on particular sensory modalities and locations. Relatively little is known about how attention to a sensory modality may relate to selection of other features, such as spatial location, in terms of brain oscillations, although it has been proposed that low-frequency modulation (α- and β-bands) may be key. Here, we investigated how attention to space (left or right) and attention to modality (vision or touch) affect ongoing low-frequency oscillatory brain activity over human sensory cortex. Magnetoencephalography was recorded while participants performed a visual or tactile task. In different blocks, touch or vision was task-relevant, whereas spatial attention was cued to the left or right on each trial. Attending to one or other modality suppressed α-oscillations over the corresponding sensory cortex. Spatial attention led to reduced α-oscillations over both sensorimotor and occipital cortex contralateral to the attended location in the cue-target interval, when either modality was task-relevant. Even modality-selective sensors also showed spatial-attention effects for both modalities. The visual and sensorimotor results were generally highly convergent, yet, although attention effects in occipital cortex were dominant in the α-band, in sensorimotor cortex, these were also clearly present in the β-band. These results extend previous findings that spatial attention can operate in a multimodal fashion and indicate that attention to space and modality both rely on similar mechanisms that modulate low-frequency oscillations.
Figures



References
-
- Bastiaansen MC, Knösche TR. Tangential derivative mapping of axial MEG applied to event-related desynchronization research. Clin Neurophysiol 111: 1300–1305, 2000 - PubMed
-
- Donner TH, Siegel M, Oostenveld R, Fries P, Bauer M, Engel AK. Population activity in the human dorsal pathway predicts the accuracy of visual motion detection. J Neurophysiol 98: 345–359, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

