Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature
- PMID: 22323721
- PMCID: PMC3566779
- DOI: 10.1523/JNEUROSCI.5606-11.2012
Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature
Abstract
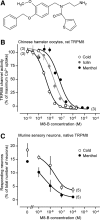
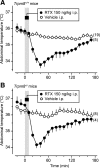
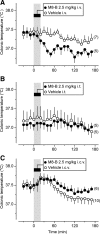
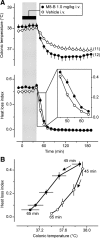
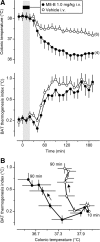
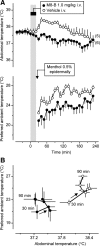
We studied N-(2-aminoethyl)-N-(4-(benzyloxy)-3-methoxybenzyl)thiophene-2-carboxamide hydrochloride (M8-B), a selective and potent antagonist of the transient receptor potential melastatin-8 (TRPM8) channel. In vitro, M8-B blocked cold-induced and TRPM8-agonist-induced activation of rat, human, and murine TRPM8 channels, including those on primary sensory neurons. In vivo, M8-B decreased deep body temperature (T(b)) in Trpm8(+/+) mice and rats, but not in Trpm8(-/-) mice, thus suggesting an on-target action. Intravenous administration of M8-B was more effective in decreasing T(b) in rats than intrathecal or intracerebroventricular administration, indicating a peripheral action. M8-B attenuated cold-induced c-Fos expression in the lateral parabrachial nucleus, thus indicating a site of action within the cutaneous cooling neural pathway to thermoeffectors, presumably on sensory neurons. A low intravenous dose of M8-B did not affect T(b) at either a constantly high or a constantly low ambient temperature (T(a)), but the same dose readily decreased T(b) if rats were kept at a high T(a) during the M8-B infusion and transferred to a low T(a) immediately thereafter. These data suggest that both a successful delivery of M8-B to the skin (high cutaneous perfusion) and the activation of cutaneous TRPM8 channels (by cold) are required for the hypothermic action of M8-B. At tail-skin temperatures <23°C, the magnitude of the M8-B-induced decrease in T(b) was inversely related to skin temperature, thus suggesting that M8-B blocks thermal (cold) activation of TRPM8. M8-B affected all thermoeffectors studied (thermopreferendum, tail-skin vasoconstriction, and brown fat thermogenesis), thus suggesting that TRPM8 is a universal cold receptor in the thermoregulation system.
Figures






References
-
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci. 2006;23:3359–3367. - PubMed
-
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Identification of temperature-sensitive neural circuits in mice using c-Fos expression mapping. Brain Res. 2003;960:157–164. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
-
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. - PubMed
-
- Boulant JA. Counterpoint: Heat-induced membrane depolarization of hypothalamic neurons: an unlikely mechanism of central thermosensitivity. Am J Physiol. 2006;290:R1481–R1484. discussion R1484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
