Distinct overlapping sequences at the carboxy-terminus of merlin regulate its tumour suppressor and morphogenic activity
- PMID: 22325036
- PMCID: PMC3822986
- DOI: 10.1111/j.1582-4934.2012.01525.x
Distinct overlapping sequences at the carboxy-terminus of merlin regulate its tumour suppressor and morphogenic activity
Abstract
The Neurofibromatosis 2 (NF2) gene product merlin is a tumour suppressor, which in addition to inhibiting cell proliferation regulates cell morphology. The morphogenic properties of merlin may play a role in tumour suppression, as patient-derived tumour cells demonstrate cytoskeletal abnormalities. However, it is still unclear how these functions are linked. The N-terminal FERM-domain of merlin is highly homologous to the oncogenic protein ezrin, while the C-termini are less conserved, suggesting that the opposite effect of the proteins on proliferation could be mediated by their distinct C-terminal regions. In this study we characterize the role of the most C-terminal residues of merlin in the regulation of proliferation, cytoskeletal organization, phosphorylation and intramolecular associations. In addition to the two full-length merlin isoforms and truncating mutations found in patients, we focused on the evolutionally conserved C-terminal residues 545-547, also harbouring disease-causing mutations. We demonstrate that merlin induces cell extensions, which result from impaired retraction of protrusions rather than from increased formation of filopodia. The residues 538-568 were found particularly important for this morphogenic activity. The results further show that both merlin isoforms are able to equally inhibit proliferation, whereas C-terminal mutants affecting residues 545-547 are less effective in growth suppression. This study demonstrates that the C-terminus contains distinct but overlapping functional domains important for regulation of the morphogenic activity, intramolecular associations and cell proliferation.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

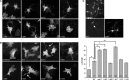


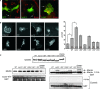


References
-
- Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–18. - PubMed
-
- Kressel M, Schmucker B. Nucleocytoplasmic transfer of the NF2 tumour suppressor protein merlin is regulated by exon 2 and a CRM1-dependent nuclear export signal in exon 15. Hum Mol Genet. 2002;11:2269–78. - PubMed
-
- Muranen T, Grönholm M, Renkema GH, et al. Cell cycle-dependent nucleocytoplasmic shuttling of the neurofibromatosis 2 tumour suppressor merlin. Oncogene. 2005;24:1150–8. - PubMed
-
- Bianchi AB, Hara T, Ramesh V, et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994;6:185–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

