Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking
- PMID: 22325201
- PMCID: PMC3345505
- DOI: 10.1016/j.neuron.2011.11.021
Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking
Abstract
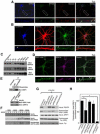
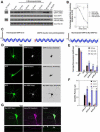
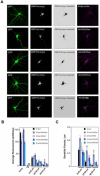
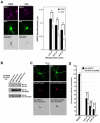
Palmitoylation, a key regulatory mechanism controlling protein targeting, is catalyzed by DHHC-family palmitoyl acyltransferases (PATs). Impaired PAT activity is linked to neurodevelopmental and neuropsychiatric disorders, suggesting critical roles for palmitoylation in neuronal function. However, few substrates for specific PATs are known, and functional consequences of palmitoylation events are frequently uncharacterized. Here, we identify the closely related PATs DHHC5 and DHHC8 as specific regulators of the PDZ domain protein GRIP1b. Binding, palmitoylation, and dendritic targeting of GRIP1b require a PDZ ligand unique to DHHC5/8. Palmitoylated GRIP1b is targeted to trafficking endosomes and may link endosomes to kinesin motors. Consistent with this trafficking role, GRIP1b's palmitoylation turnover rate approaches the highest of all reported proteins, and palmitoylation increases GRIP1b's ability to accelerate AMPA-R recycling. To our knowledge, these findings identify the first neuronal DHHC5/8 substrate, define novel mechanisms controlling palmitoylation specificity, and suggest further links between dysregulated palmitoylation and neuropathological conditions.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Chen WY, Shi YY, Zheng YL, Zhao XZ, Zhang GJ, Chen SQ, Yang PD, He L. Case-control study and transmission disequilibrium test provide consistent evidence for association between schizophrenia and genetic variation in the 22q11 gene ZDHHC8. Hum. Mol. Genet. 2005;13:2991–2995. - PubMed
-
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–876. - PubMed
-
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

