Different cell fates from cell-cell interactions: core architectures of two-cell bistable networks
- PMID: 22325263
- PMCID: PMC3274795
- DOI: 10.1016/j.bpj.2011.11.4022
Different cell fates from cell-cell interactions: core architectures of two-cell bistable networks
Abstract
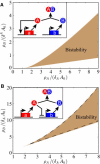
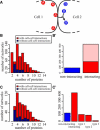
The acquisition of different fates by cells that are initially in the same state is central to development. Here, we investigate the possible structures of bistable genetic networks that can allow two identical cells to acquire different fates through cell-cell interactions. Cell-autonomous bistable networks have been previously sampled using an evolutionary algorithm. We extend this evolutionary procedure to take into account interactions between cells. We obtain a variety of simple bistable networks that we classify into major subtypes. Some have long been proposed in the context of lateral inhibition through the Notch-Delta pathway, some have been more recently considered and others appear to be new and based on mechanisms not previously considered. The results highlight the role of posttranscriptional interactions and particularly of protein complexation and sequestration, which can replace cooperativity in transcriptional interactions. Some bistable networks are entirely based on posttranscriptional interactions and the simplest of these is found to lead, upon a single parameter change, to oscillations in the two cells with opposite phases. We provide qualitative explanations as well as mathematical analyses of the dynamical behaviors of various created networks. The results should help to identify and understand genetic structures implicated in cell-cell interactions and differentiation.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Wilkinson H.A., Fitzgerald K., Greenwald I. Reciprocal changes in expression of the receptor lin-12 and its ligand lag-2 prior to commitment in a C. elegans cell fate decision. Cell. 1994;79:1187–1198. - PubMed
-
- Heitzler P., Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. - PubMed
-
- Henrique D., Adam J., Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

