The role of lipids in VDAC oligomerization
- PMID: 22325275
- PMCID: PMC3274789
- DOI: 10.1016/j.bpj.2011.12.049
The role of lipids in VDAC oligomerization
Abstract
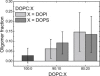
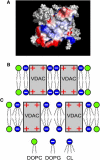
Evidence has accumulated that the voltage-dependent anion channel (VDAC), located on the outer membrane of mitochondria, plays a central role in apoptosis. The involvement of VDAC oligomerization in apoptosis has been suggested in various studies. However, it still remains unknown how exactly VDAC supramolecular assembly can be regulated in the membrane. This study addresses the role of lipids in this process. We investigate the effect of cardiolipin (CL) and phosphatidylglycerol (PG), anionic lipids important for mitochondria metabolism and apoptosis, on VDAC oligomerization. By applying fluorescence cross-correlation spectroscopy to VDAC reconstituted into giant unilamellar vesicles, we demonstrate that PG significantly enhances VDAC oligomerization in the membrane, whereas cardiolipin disrupts VDAC supramolecular assemblies. During apoptosis, the level of PG in mitochondria increases, whereas the CL level decreases. We suggest that the specific lipid composition of the outer mitochondrial membrane might be of crucial relevance and, thus, a potential cue for regulating the oligomeric state of VDAC.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


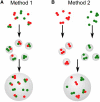
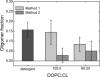
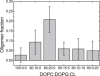
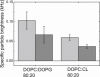
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

