Mapping the nerve architecture of diabetic human corneas
- PMID: 22325488
- PMCID: PMC3480080
- DOI: 10.1016/j.ophtha.2011.10.036
Mapping the nerve architecture of diabetic human corneas
Abstract
Objective: To investigate the entire human corneal nerve architecture of donors with different durations of insulin-dependent diabetes mellitus (IDDM).
Design: Experimental study.
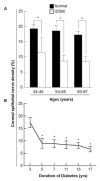
Participants and controls: Sixteen fresh human eyes from 8 diabetic donors (aged 43-66 years, with IDDM for 2-17 years) and 12 eyes from 6 normal donors (aged from 44-67 years) were obtained from the National Disease Research Interchange (NDRI).
Methods: After fixation, corneas were stained with mouse monoclonal anti-β-Tubulin III antibody, and images were acquired to build a whole view of the corneal nerve architecture. The same corneas were used for both whole-mount and cross-section examination.
Main outcome measures: Corneal epithelial nerve density was calculated on the basis of the whole-mount view of the central area. The number of stromal nerves was calculated by counting the nerve trunks at the corneoscleral limbus of the entire cornea. Differences between diabetic and normal corneas in epithelial nerve densities and main stromal nerve numbers were compared by paired-samples t test.
Results: The diabetic eyes presented numerous neuropathies in areas where the epithelial nerve bundles emerged. A striking pathologic change was the presence of abundant nerve fiber loops in the stroma. These loops seemed to form by resistance presented by the basement membrane, which may prevent penetration of stromal nerve branches into epithelia. There was no difference in the numbers of main stromal nerve trunks between corneas from diabetic and normal donors, but there was a significant decrease in central epithelial nerve density in the diabetic corneas. We did not find an age effect on this decrease. Instead, it was significantly affected by 5 or more years of IDDM.
Conclusions: This is the first study to show an entire view of the nerve architecture in human diabetic corneas. The decreased epithelial nerve density may result from the abnormalities of stromal nerve architecture and is affected by 5 or more years of IDDM. Although compensation for some nerve regeneration takes place, the alterations in the stromal nerves can explain the poor healing and persistent epithelial defects seen in diabetic patients.
Copyright © 2012 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, content and function. Exp Eye Res. 2003;76:521–42. - PubMed
-
- Riaz SS, Tomlinson DR. Neurotrophic factors in peripheral neuropathies: pharmacological strategies. Prog Neurobiol. 1996;49:125–43. - PubMed
-
- Kingsley RE, Marfurt CF. Topical substance P and corneal epithelial wound closure in the rabbit. Invest Ophthalmol Vis Sci. 1997;38:388–95. - PubMed
-
- Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66:421–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

