Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1
- PMID: 22326219
- PMCID: PMC3278712
- DOI: 10.1016/j.cmet.2012.01.004
Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1
Abstract
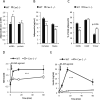
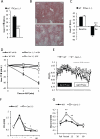
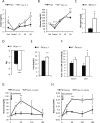
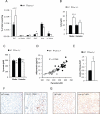
Caveolin-1 is a major structural component of raft structures within the plasma membrane and has been implicated as a regulator of cellular signal transduction with prominent expression in adipocytes. Here, we embarked on a comprehensive characterization of the metabolic pathways dysregulated in caveolin-1 null mice. We found that these mice display decreased circulating levels of total and high molecular weight adiponectin and a reduced ability to change substrate use in response to feeding/fasting conditions. Caveolin-1 null mice are extremely lean but retain muscle mass despite lipodystrophy and massive metabolic dysfunction. Hepatic gluconeogenesis is chronically elevated, while hepatic steatosis is reduced. Our data suggest that the complex phenotype of the caveolin-1 null mouse is caused by altered metabolic and mitochondrial function in adipose tissue with a subsequent compensatory response driven mostly by the liver. This mouse model highlights the central contributions of adipose tissue for system-wide preservation of metabolic flexibility.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. - PMC - PubMed
-
- Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. The J Biol Chem. 2007;282:31257–31266. - PubMed
-
- Babich H, Liebling EJ, Burger RF, Zuckerbraun HL, Schuck AG. Choice of DMEM, formulated with or without pyruvate, plays an important role in assessing the in vitro cytotoxicity of oxidants and prooxidant nutraceuticals. In Vitro Cell Dev Biol Anim. 2009;45:226–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

