The HMGN family of chromatin-binding proteins: dynamic modulators of epigenetic processes
- PMID: 22326857
- PMCID: PMC3371129
- DOI: 10.1016/j.bbagrm.2012.01.013
The HMGN family of chromatin-binding proteins: dynamic modulators of epigenetic processes
Abstract
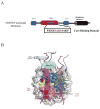
The HMGN family of proteins binds to nucleosomes without any specificity for the underlying DNA sequence. They affect the global and local structure of chromatin, as well as the levels of histone modifications and thus play a role in epigenetic regulation of gene expression. This review focuses on the recent studies that provide new insights on the interactions between HMGN proteins, nucleosomes, and chromatin, and the effects of these interactions on epigenetic and transcriptional regulation. This article is part of a Special Issue entitled: Chromatin in time and space.
Published by Elsevier B.V.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

