Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice
- PMID: 22326951
- PMCID: PMC3287230
- DOI: 10.1172/JCI60329
Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice
Abstract
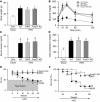
The leading cause of death in diabetic patients is cardiovascular disease; diabetic cardiomyopathy is typified by alterations in cardiac morphology and function, independent of hypertension or coronary disease. However, the molecular mechanism that links diabetes to cardiomyopathy is incompletely understood. Insulin resistance is a hallmark feature of diabetes, and the FoxO family of transcription factors, which regulate cell size, viability, and metabolism, are established targets of insulin and growth factor signaling. Here, we set out to evaluate a possible role of FoxO proteins in diabetic cardiomyopathy. We found that FoxO proteins were persistently activated in cardiac tissue in mice with diabetes induced either genetically or by high-fat diet (HFD). FoxO activity was critically linked with development of cardiomyopathy: cardiomyocyte-specific deletion of FoxO1 rescued HFD-induced declines in cardiac function and preserved cardiomyocyte insulin responsiveness. FoxO1-depleted cells displayed a shift in their metabolic substrate usage, from free fatty acids to glucose, associated with decreased accumulation of lipids in the heart. Furthermore, we found that FoxO1-dependent downregulation of IRS1 resulted in blunted Akt signaling and insulin resistance. Together, these data suggest that activation of FoxO1 is an important mediator of diabetic cardiomyopathy and is a promising therapeutic target for the disease.
Figures
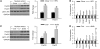





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

