The intersection of genetic and chemical genomic screens identifies GSK-3α as a target in human acute myeloid leukemia
- PMID: 22326953
- PMCID: PMC3287215
- DOI: 10.1172/JCI46465
The intersection of genetic and chemical genomic screens identifies GSK-3α as a target in human acute myeloid leukemia
Abstract
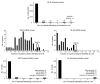
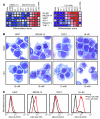
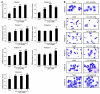
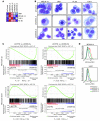
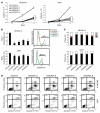
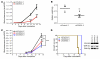
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults. Long-term survival of patients with AML has changed little over the past decade, necessitating the identification and validation of new AML targets. Integration of genomic approaches with small-molecule and genetically based high-throughput screening holds the promise of improved discovery of candidate targets for cancer therapy. Here, we identified a role for glycogen synthase kinase 3α (GSK-3α) in AML by performing 2 independent small-molecule library screens and an shRNA screen for perturbations that induced a differentiation expression signature in AML cells. GSK-3 is a serine-threonine kinase involved in diverse cellular processes, including differentiation, signal transduction, cell cycle regulation, and proliferation. We demonstrated that specific loss of GSK-3α induced differentiation in AML by multiple measurements, including induction of gene expression signatures, morphological changes, and cell surface markers consistent with myeloid maturation. GSK-3α-specific suppression also led to impaired growth and proliferation in vitro, induction of apoptosis, loss of colony formation in methylcellulose, and anti-AML activity in vivo. Although the role of GSK-3β has been well studied in cancer development, these studies support a role for GSK-3α in AML.
Figures

References
-
- Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2010;24(1):35–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

