IAPs limit activation of RIP kinases by TNF receptor 1 during development
- PMID: 22327219
- PMCID: PMC3321198
- DOI: 10.1038/emboj.2012.18
IAPs limit activation of RIP kinases by TNF receptor 1 during development
Abstract
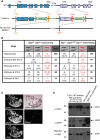
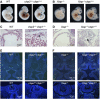
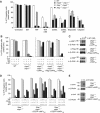
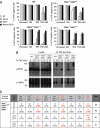
Inhibitor of apoptosis (IAP) proteins cIAP1, cIAP2, and XIAP (X-linked IAP) regulate apoptosis and cytokine receptor signalling, but their overlapping functions make it difficult to distinguish their individual roles. To do so, we deleted the genes for IAPs separately and in combination. While lack of any one of the IAPs produced no overt phenotype in mice, deletion of cIap1 with cIap2 or Xiap resulted in mid-embryonic lethality. In contrast, Xiap(-/-)cIap2(-/-) mice were viable. The death of cIap2(-/-)cIap1(-/-) double mutants was rescued to birth by deletion of tumour necrosis factor (TNF) receptor 1, but not TNFR2 genes. Remarkably, hemizygosity for receptor-interacting protein kinase 1 (Ripk1) allowed Xiap(-/-)cIap1(-/-) double mutants to survive past birth, and prolonged cIap2(-/-)cIap1(-/-) embryonic survival. Similarly, deletion of Ripk3 was able to rescue the mid-gestation defect of cIap2(-/-)cIap1(-/-) embryos, as these embryos survived to E15.5. cIAPs are therefore required during development to limit activity of RIP kinases in the TNF receptor 1 signalling pathway.
Conflict of interest statement
DLV and JS are on the Scientific Advisory Board of TetraLogic Pharmaceuticals.
Figures



Comment in
-
cIAP2 supports viability of mice lacking cIAP1 and XIAP.EMBO J. 2015 Oct 1;34(19):2393-5. doi: 10.15252/embj.201592060. EMBO J. 2015. PMID: 26427758 Free PMC article. No abstract available.
-
Response to Heard et al.EMBO J. 2015 Oct 1;34(19):2396-7. doi: 10.15252/embj.201592761. EMBO J. 2015. PMID: 26427759 Free PMC article. No abstract available.
References
-
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30: 689–700 - PubMed
-
- Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P (2006) Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem 281: 13964–13971 - PubMed
-
- Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL (2003) Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8: 345–352 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

