Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function
- PMID: 22327427
- PMCID: PMC3338938
- DOI: 10.4161/mabs.4.1.18347
Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function
Abstract
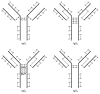
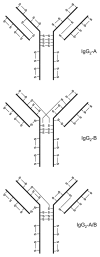
The disulfide bond structures established decades ago for immunoglobulins have been challenged by findings from extensive characterization of recombinant and human monoclonal IgG antibodies. Non-classical disulfide bond structure was first identified in IgG4 and later in IgG2 antibodies. Although, cysteine residues should be in the disulfide bonded states, free sulfhydryls have been detected in all subclasses of IgG antibodies. In addition, disulfide bonds are susceptible to chemical modifications, which can further generate structural variants such as IgG antibodies with trisulfide bond or thioether linkages. Trisulfide bond formation has also been observed for IgG of all subclasses. Degradation of disulfide bond through β-elimination generates free sulfhydryls disulfide and dehydroalanine. Further reaction between free sulfhydryl and dehydroalanine leads to the formation of a non-reducible cross-linked species. Hydrolysis of the dehydroalanine residue contributes substantially to antibody hinge region fragmentation. The effect of these disulfide bond variations on antibody structure, stability and biological function are discussed in this review.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources