Analytical characterization of ch14.18: a mouse-human chimeric disialoganglioside-specific therapeutic antibody
- PMID: 22327432
- PMCID: PMC3338943
- DOI: 10.4161/mabs.4.1.18566
Analytical characterization of ch14.18: a mouse-human chimeric disialoganglioside-specific therapeutic antibody
Abstract
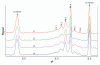
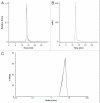
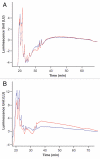
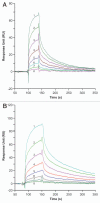
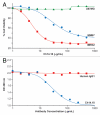
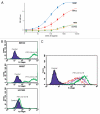
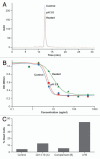
Ch14.18 is a mouse-human chimeric monoclonal antibody to the disialoganglioside (GD2) glycolipid. In the clinic, this antibody has been shown to be effective in the treatment of children with high-risk neuroblastoma, either alone or in combination therapy. Extensive product characterization is a prerequisite to addressing the potential issues of product variability associated with process changes and manufacturing scale-up. Charge heterogeneity, glycosylation profile, molecular state and aggregation, interaction (affinity) with Fcγ receptors and functional or biological activities are a few of the critical characterization assays for assessing product comparability for this antibody. In this article, we describe the in-house development and qualification of imaged capillary isoelectric focusing to assess charge heterogeneity, analytical size exclusion chromatography with online static and dynamic light scattering (DLS), batch mode DLS for aggregate detection, biosensor (surface plasmon resonance)-based Fcγ receptor antibody interaction kinetics, N-glycoprofiling with PNGase F digestion, 2-aminobenzoic acid labeling and high performance liquid chromatography and N-glycan analysis using capillary electrophoresis. In addition, we studied selected biological activity assays, such as complement-dependent cytotoxicity. The consistency and reproducibility of the assays are established by comparing the intra-day and inter-day assay results. Applications of the methodologies to address stability or changes in product characteristics are also reported. The study results reveal that the ch14.18 clinical product formulated in phosphate-buffered saline at a concentration of 5 mg/ml and stored at 2-8°C is stable for more than five years.
Figures

References
-
- Shapiro MA, Swann PG, Hartsough M. Regulatory considerations in the development of monoclonal antibodies for diagnosis and therapy. In: Dubel S, editor. Handbook of Therapeutic Antibodies. Weinham, Germany: Wiley VCH; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
