Serum albumin targeted, pH-dependent magnetic resonance relaxation agents
- PMID: 22328098
- PMCID: PMC3304010
- DOI: 10.1002/chem.201103344
Serum albumin targeted, pH-dependent magnetic resonance relaxation agents
Abstract
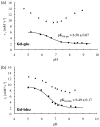
The objective of this work was the synthesis of serum albumin targeted, Gd(III)-based magnetic resonance imaging (MRI) contrast agents exhibiting a strong pH-dependent relaxivity. Two new complexes (Gd-glu and Gd-bbu) were synthesized based on the DO3A macrocycle modified with three carboxyalkyl substituents α to the three ring nitrogen atoms, and a biphenylsulfonamide arm. The sulfonamide nitrogen coordinates the Gd in a pH-dependent fashion, resulting in a decrease in the hydration state, q, as pH is increased and a resultant decrease in relaxivity (r(1)). In the absence of human serum albumin (HSA), r(1) increases from 2.0 to 6.0 mM(-1) s(-1) for Gd-glu and from 2.4 to 9.0 mM(-1) s(-1) for Gd-bbu from pH 5 to 8.5 at 37 °C, 0.47 T, respectively. These complexes (0.2 mM) are bound (>98.9 %) to HSA (0.69 mM) over the pH range 5-8.5. Binding to albumin increases the rotational correlation time and results in higher relaxivity. The r(1) increased 120 % (pH 5) and 550 % (pH 8.5) for Gd-glu and 42 % (pH 5) and 260 % (pH 8.5) for Gd-bbu. The increases in r(1) at pH 5 were unexpectedly low for a putative slow tumbling q=2 complex. The Gd-bbu system was investigated further. At pH 5, it binds in a stepwise fashion to HSA with dissociation constants K(d1)=0.65, K(d2)=18, K(d3)=1360 μM. The relaxivity at each binding site was constant. Luminescence lifetime titration experiments with the Eu(III) analogue revealed that the inner-sphere water ligands are displaced when the complex binds to HSA resulting in lower than expected r(1) at pH 5. Variable pH and temperature nuclear magnetic relaxation dispersion (NMRD) studies showed that the increased r(1) of the albumin-bound q=0 complexes is due to the presence of a nearby water molecule with a long residency time (1-2 ns). The distance between this water molecule and the Gd ion changes with pH resulting in albumin-bound pH-dependent relaxivity.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Tomlinson FH, Anderson RE, Meyer FB. Stroke. 1993;24:2030–2039. discussion 2040. - PubMed
-
- Buxton RB, Wechsler LR, Alpert NM, Ackerman RH, Elmaleh DR, Correia JA. J Cereb Blood Flow Metab. 1984;4:8–16. - PubMed
- Garcia-Martin ML, Herigault G, Remy C, Farion R, Ballesteros P, Coles JA, Cerdan S, Ziegler A. Cancer Res. 2001;61:6524–6531. - PubMed
- Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. J Magn Reson Imaging. 2002;16:430–450. - PubMed
- Zhang X, Lin Y, Gillies RJ. J Nucl Med. 2010;51:1167–1170. - PMC - PubMed
-
- Lowe M, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E, Pagliarin R. J Am Chem Soc. 2001;123:7601–7609. - PubMed
- Woods M, Kiefer GE, Bott S, Castillo-Muzquiz A, Eshelbrenner C, Michaudet L, McMillan K, Mudigunda SDK, Ogrin D, Tircsó G, Zhang S, Zhao P, Sherry AD. J Am Chem Soc. 2004;126:9248–9256. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

