Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection
- PMID: 22328294
- PMCID: PMC3358510
- DOI: 10.1002/lt.23411
Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection
Abstract
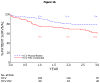
Hepatitis C virus (HCV) is a controversial indication for liver transplantation (LT) in human immunodeficiency virus (HIV)-infected patients because of reportedly poor outcomes. This prospective, multicenter US cohort study compared patient and graft survival for 89 HCV/HIV-coinfected patients and 2 control groups: 235 HCV-monoinfected LT controls and all US transplant recipients who were 65 years old or older. The 3-year patient and graft survival rates were 60% [95% confidence interval (CI) = 47%-71%] and 53% (95% CI = 40%-64%) for the HCV/HIV patients and 79% (95% CI = 72%-84%) and 74% (95% CI = 66%-79%) for the HCV-infected recipients (P < 0.001 for both), and HIV infection was the only factor significantly associated with reduced patient and graft survival. Among the HCV/HIV patients, older donor age [hazard ratio (HR) = 1.3 per decade], combined kidney-liver transplantation (HR = 3.8), an anti-HCV-positive donor (HR = 2.5), and a body mass index < 21 kg/m(2) (HR = 3.2) were independent predictors of graft loss. For the patients without the last 3 factors, the patient and graft survival rates were similar to those for US LT recipients. The 3-year incidence of treated acute rejection was 1.6-fold higher for the HCV/HIV patients versus the HCV patients (39% versus 24%, log rank P = 0.02), but the cumulative rates of severe HCV disease at 3 years were not significantly different (29% versus 23%, P = 0.21). In conclusion, patient and graft survival rates are lower for HCV/HIV-coinfected LT patients versus HCV-monoinfected LT patients. Importantly, the rates of treated acute rejection (but not the rates of HCV disease severity) are significantly higher for HCV/HIV-coinfected recipients versus HCV-infected recipients. Our results indicate that HCV per se is not a contraindication to LT in HIV patients, but recipient and donor selection and the management of acute rejection strongly influence outcomes.
Copyright © 2012 American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures



Comment in
-
Is liver transplantation feasible in patients coinfected with human immunodeficiency virus and hepatitis C virus?Liver Transpl. 2012 Jun;18(6):744-5; author reply 746. doi: 10.1002/lt.23419. Liver Transpl. 2012. PMID: 22359380 No abstract available.
-
Liver transplantation: outcomes could be improved in HCV-HIV co-infected liver transplant recipients.Nat Rev Gastroenterol Hepatol. 2012 Mar 6;9(4):190. doi: 10.1038/nrgastro.2012.25. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22392293 No abstract available.
-
Liver transplantation in human immunodeficiency virus/hepatitis C virus-coinfected patients: response needed!Liver Transpl. 2012 Jun;18(6):617-8. doi: 10.1002/lt.23431. Liver Transpl. 2012. PMID: 22431195 No abstract available.
-
Liver transplantation in the human immunodeficiency virus-hepatitis C virus coinfected patient: time to sum up.Hepatology. 2013 Jan;57(1):409-11. doi: 10.1002/hep.26123. Hepatology. 2013. PMID: 23297070 No abstract available.
References
-
- Lesens O, Deschenes M, Steben M, Belanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179:1254–8. - PubMed
-
- Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. - PubMed
-
- Roland ME, Barin B, Carlson L, Frassetto LA, Terrault NA, Hirose R, Freise CE, Benet LZ, Ascher NL, Roberts JP, Murphy B, Keller MJ, Olthoff KM, Blumberg EA, Brayman KL, Bartlett ST, Davis CE, McCune JM, Bredt BM, Stablein DM, Stock PG. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8:355–65. - PubMed
-
- de Vera ME, Dvorchik I, Tom K, Eghtesad B, Thai N, Shakil O, Marcos A, Demetris A, Jain A, Fung JJ, Ragni MV. Survival of Liver Transplant Patients Coinfected with HIV and HCV Is Adversely Impacted by Recurrent Hepatitis C. Am J Transplant. 2006;6:2983–2993. - PubMed
-
- Duclos-Vallee JC, Feray C, Sebagh M, Teicher E, Roque-Afonso AM, Roche B, Azoulay D, Adam R, Bismuth H, Castaing D, Vittecoq D, Samuel D. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47:407–17. - PubMed
