Spry1 and spry2 are essential for development of the temporomandibular joint
- PMID: 22328578
- PMCID: PMC3310757
- DOI: 10.1177/0022034512438401
Spry1 and spry2 are essential for development of the temporomandibular joint
Abstract
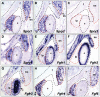
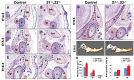
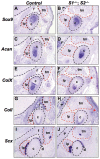
The temporomandibular joint (TMJ) is a specialized synovial joint essential for the function of the mammalian jaw. The main components of the TMJ are the mandibular condyle, the glenoid fossa of the temporal bone, and a fibrocartilagenous disc interposed between them. The genetic program for the development of the TMJ remains poorly understood. Here we show the crucial role of sprouty (Spry) genes in TMJ development. Sprouty genes encode intracellular inhibitors of receptor tyrosine kinase (RTK) signaling pathways, including those triggered by fibroblast growth factors (Fgfs). Using in situ hybridization, we show that Spry1 and Spry2 are highly expressed in muscles attached to the TMJ, including the lateral pterygoid and temporalis muscles. The combined inactivation of Spry1 and Spry2 results in overgrowth of these muscles, which disrupts normal development of the glenoid fossa. Remarkably, condyle and disc formation are not affected in these mutants, demonstrating that the glenoid fossa is not required for development of these structures. Our findings demonstrate the importance of regulated RTK signaling during TMJ development and suggest multiple skeletal origins for the fossa. Notably, our work provides the evidence that the TMJ condyle and disc develop independently of the mandibular fossa.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Armand AS, Laziz I, Chanoine C. (2006). FGF6 in myogenesis. Biochim Biophys Acta 1763:773-778 - PubMed
-
- Avery JK. (2001). Oral development and histology. 3rd ed. New York, NY: Thieme Medical Publishers
-
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, et al. (2005). Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8:229-239 - PubMed
-
- Brent AE, Tabin CJ. (2004). FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131:3885-3896 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

