Cell-to-cell signaling influences the fate of prostate cancer stem cells and their potential to generate more aggressive tumors
- PMID: 22328933
- PMCID: PMC3273473
- DOI: 10.1371/journal.pone.0031467
Cell-to-cell signaling influences the fate of prostate cancer stem cells and their potential to generate more aggressive tumors
Abstract
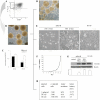
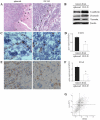
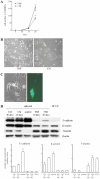
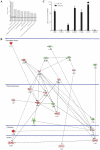
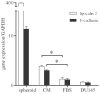
An increasing number of malignancies has been shown to be initiated and propelled by small subpopulations of cancer stem cells (CSC). However, whether tumor aggressiveness is driven by CSC and by what extent this property may be relevant within the tumor mass is still unsettled. To address this issue, we isolated a rare tumor cell population on the basis of its CD44(+)CD24(-) phenotype from the human androgen-independent prostate carcinoma cell line DU145 and established its CSC properties. The behavior of selected CSC was investigated with respect to the bulk DU145 cells. The injection of CSC in nude mice generated highly vascularized tumors infiltrating the adjacent tissues, showing high density of neuroendocrine cells and expressing low levels of E-cadherin and β-catenin as well as high levels of vimentin. On the contrary, when a comparable number of unsorted DU145 cells were injected the resulting tumors were less aggressive. To investigate the different features of tumors in vivo, the influence of differentiated tumor cells on CSC was examined in vitro by growing CSC in the absence or presence of conditioned medium from DU145 cells. CSC grown in permissive conditions differentiated into cell populations with features similar to those of cells held in aggressive tumors generated from CSC injection. Differently, conditioned medium induced CSC to differentiate into a cell phenotype comparable to cells of scarcely aggressive tumors originated from bulk DU145 cell injection. These findings show for the first time that CSC are able to generate differentiated cells expressing either highly or scarcely aggressive phenotype, thus influencing prostate cancer progression. The fate of CSC was determined by signals released from tumor environment. Moreover, using microarray analysis we selected some molecules which could be involved in this cell-to-cell signaling, hypothesizing their potential value for prognostic or therapeutic applications.
Conflict of interest statement
Figures


References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. - PubMed
-
- Bonkhoff H, Stein U, Remberger K. Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol. 1993;423:291–294. - PubMed
-
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. - PubMed
-
- Abrahamsson PA, Di Sant'Agnese PA. Neuroendocrine cells in the human prostate gland. J Androl. 1993;14:307–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

