Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer
- PMID: 22328973
- PMCID: PMC3274752
- DOI: 10.1158/2159-8274.CD-11-0005
Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer
Abstract
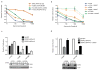
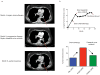
While genomically targeted therapies have improved outcomes for patients with lung adenocarcinoma, little is known about the genomic alterations which drive squamous cell lung cancer. Sanger sequencing of the tyrosine kinome identified mutations in the DDR2 kinase gene in 3.8% of squamous cell lung cancers and cell lines. Squamous lung cancer cell lines harboring DDR2 mutations were selectively killed by knock-down of DDR2 by RNAi or by treatment with the multi-targeted kinase inhibitor dasatinib. Tumors established from a DDR2 mutant cell line were sensitive to dasatinib in xenograft models. Expression of mutated DDR2 led to cellular transformation which was blocked by dasatinib. A squamous cell lung cancer patient with a response to dasatinib and erlotinib treatment harbored a DDR2 kinase domain mutation. These data suggest that gain-of-function mutations in DDR2 are important oncogenic events and are amenable to therapy with dasatinib. As dasatinib is already approved for use, these findings could be rapidly translated into clinical trials.
Significance: DDR2 mutations are present in 4% of lung SCCs, and DDR2 mutations are associated with sensitivity to dasatinib. These findings provide a rationale for designing clinical trials with the FDA-approved drug dasatinib in patients with lung SCCs.
Keywords: DDR2; Squamous cell lung cancer; dasatinib; lung cancer genomics; tyrosine kinase inhibitors.
Conflict of interest statement
Conflicts of Interest: M.M. is a consultant to Novartis and receives research support from Novartis, receives research support from Genentech, is a founding advisor and consultant to, and an equity holder in, Foundation Medicine and patent holder for EGFR mutation testing, licensed to Genzyme Genetics. N.S.G. receives research support from Novartis. E B.H. was the principal investigator of an industry-sponsored clinical trial of dasatinib and erlotinib in lung cancer funded in part by Bristol-Myers-Squibb and the American Society for Clinical Oncology. R.K.T. reports consulting and lecture fees (Sequenom, Sanofi-Aventis, Merck, Roche, Infinity, Boehringer, Astra-Zeneca, Johnson&Johnson, Atlas-Biolabs) and research support (Novartis, AstraZeneca).
Figures



Comment in
-
A new target for therapy in squamous cell carcinoma of the lung.Cancer Discov. 2011 Jun;1(1):23-4. doi: 10.1158/2159-8274.CD-11-0069. Epub 2011 Apr 14. Cancer Discov. 2011. PMID: 22586316
References
-
- Lennes IT, Lynch TJ. Quality indicators in cancer care: development and implementation for improved health outcomes in non-small-cell lung cancer. Clin Lung Cancer. 2009;10(5):341–6. - PubMed
-
- West H, Harpole D, Travis W. Histologic considerations for individualized systemic therapy approaches for the management of non-small cell lung cancer. Chest. 2009;136(4):1112–8. - PubMed
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

