Genomic complexity and AKT dependence in serous ovarian cancer
- PMID: 22328975
- PMCID: PMC3274821
- DOI: 10.1158/2159-8290.CD-11-0170
Genomic complexity and AKT dependence in serous ovarian cancer
Erratum in
- Cancer Discov. 2012 Apr;2(4):376. Janikariman, Manickam [corrected to Janakiraman, Manickam]
Abstract
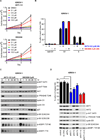
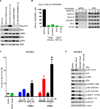
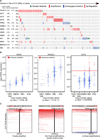
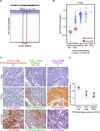
Effective oncoprotein-targeted therapies have not yet been developed for ovarian cancer. To explore the role of PI3 kinase/AKT signaling in this disease, we performed a genetic and functional analysis of ovarian cancer cell lines and tumors. PI3K pathway alterations were common in both, but the spectrum of mutational changes differed. Genetic activation of the pathway was necessary, but not sufficient, to confer sensitivity to selective inhibition of AKT and cells with RAS pathway alterations or RB1 loss were resistant to AKT inhibition, whether or not they had coexistent PI3K/AKT pathway activation. Inhibition of AKT1 caused growth arrest in a subset of ovarian cell lines, but not in those with AKT3 expression, which required pan-AKT inhibition. Thus, a subset of ovarian tumors are sensitive to AKT inhibition, but the genetic heterogeneity of the disease suggests that effective treatment with AKT pathway inhibitors will require a detailed molecular analysis of each patient's tumor.
Significance: A subset of ovarian cancers exhibits AKT pathway activation and is sensitive to selective AKT inhibition. Ovarian tumors exhibit significant genetic heterogeneity and thus an individualized approach based on real-time, detailed genomic and proteomic characterization of individual tumors will be required for the successful application of PI3K/AKT pathway inhibitors in this disease.
Keywords: AKT; MK2206; Ovarian; PTEN; TCGA.
Figures


Comment in
-
Dissecting "PI3Kness": the complexity of personalized therapy for ovarian cancer.Cancer Discov. 2012 Jan;2(1):16-8. doi: 10.1158/2159-8290.CD-11-0323. Cancer Discov. 2012. PMID: 22585163 Free PMC article.
References
-
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. - PubMed
-
- Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

