Transthyretin cardiac amyloidoses in older North Americans
- PMID: 22329529
- PMCID: PMC3325376
- DOI: 10.1111/j.1532-5415.2011.03868.x
Transthyretin cardiac amyloidoses in older North Americans
Abstract
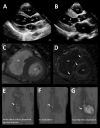
The amyloidoses are a group of hereditary or acquired disorders caused by the extracellular deposition of insoluble protein fibrils that impair tissue structure and function. All amyloidoses result from protein misfolding, a common mechanism for disorders in older persons, including Alzheimer's disease and Parkinson's disease. Abnormalities in the protein transthyretin (TTR), a serum transporter of thyroxine and retinol, is the most common cause of cardiac amyloidoses in elderly adults. Mutations in TTR can result in familial amyloidotic cardiomyopathy, and wild-type TTR can result in senile cardiac amyloidosis. These underdiagnosed disorders are much more common than previously thought. The resulting restrictive cardiomyopathy can cause congestive heart failure, arrhythmias, and advanced conduction system disease. Although historically difficult to make, the diagnosis of TTR cardiac amyloidosis has become easier in recent years with advances in cardiac imaging and more widespread use of genetic analysis. Although therapy has largely involved supportive medical care, avoidance of potentially toxic agents, and rarely organ transplantation, the near future brings the possibility of targeted pharmacotherapies designed to prevent TTR misfolding and amyloid deposition. Because these disease-modifying agents are designed to prevent disease progression, it has become increasingly important that older persons with TTR amyloidosis be expeditiously identified and considered for enrollment in clinical registries and trials.
© 2012, Copyright the Authors Journal compilation © 2012, The American Geriatrics Society.
Figures


References
-
- Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: A comprehensive review. Arch Intern Med. 2006;166:1805–1813. - PubMed
-
- Desai HV, Aronow WS, Peterson SJ, et al. Cardiac amyloidosis: Approaches to diagnosis and management. Cardiol Rev. 2010;18:1–11. - PubMed
-
- Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: A clinical overview. Nat Rev Cardiol. 2010;7:398–408. - PubMed
-
- Westermark P, Benson MD, Buxbaum JN, et al. Amyloid: toward terminology clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2005;12:1–4. - PubMed
-
- Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52:347–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

