The effect of budesonide/formoterol pressurized metered-dose inhaler on predefined criteria for worsening asthma in four different patient populations with asthma
- PMID: 22329608
- PMCID: PMC3586061
- DOI: 10.2165/11630600-000000000-00000
The effect of budesonide/formoterol pressurized metered-dose inhaler on predefined criteria for worsening asthma in four different patient populations with asthma
Erratum in
- Drugs R D. 2012 Mar 1;12(1):15
Abstract
Background: Previous studies have shown disparities between Black and Hispanic patients compared with other populations in response to asthma medications.
Objective: The aim of this analysis was to assess the effect of budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI) and BUD on predefined criteria for asthma worsening, an asthma control metric generally aligned with definitions of moderate asthma exacerbations, across four different populations.
Methods: Data were from four 12-week, randomized, double-blind, US studies of BUD/FM pMDI treatment in patients aged 12 years or older with varying asthma severities and of varying races. Predefined asthma events and withdrawals due to predefined events were assessed as secondary study endpoints. Study I (NCT00651651) includes data from predominantly White patients with mild to moderate asthma who were randomized to BUD/FM pMDI 160/9 μg twice daily (bid; n = 123) or BUD pMDI 160 μg bid (n = 121). Study II (NCT00652002) includes data from predominantly White patients with moderate to severe asthma who were randomized to BUD/FM pMDI 320/9 μg bid (n = 124) or BUD pMDI 320 μg bid (n = 109). Study III (NCT00702325) included self-reported Black patients with moderate to severe asthma who were randomized to BUD/FM pMDI 320/9 μg bid (n = 153) or BUD dry powder inhaler 360 μg bid (n = 148). Study IV (NCT00419757) included self-reported Hispanic patients with moderate to severe asthma who were randomized to BUD/FM pMDI 320/9 μg bid (n = 127) or BUD pMDI 320 μg bid (n = 123). Patients were to be withdrawn from the studies if they developed an asthma event, as determined by predefined criteria, except for night-time awakenings, where withdrawal was left to the study physician's judgment.
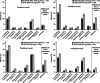
Results: Overall, fewer patients in the studies (study I, II, III, and IV, respectively) experienced ≥1 asthma event in the BUD/FM group (18.7%, 29.8%, 37.3%, 25.2%) versus the BUD group (21.5%, 44.0%, 45.3%, 31.7%); only study II results showed a statistically significant difference between treatments. Fewer patients with moderate to severe asthma (studies II, III, and IV) were withdrawn due to ≥1 asthma event in the BUD/FM group (10.5%, 11.8%, 3.1%, respectively) than in the BUD group (20.2%, 18.9%, 6.5%, respectively); however, percentages were similar in the BUD/FM (7.3%) and BUD (6.6%) groups in patients with mild to moderate asthma (study I).
Conclusions: Predefined asthma event rates were numerically or significantly lower for patients with asthma receiving BUD/FM pMDI versus BUD, regardless of race or disease severity. Differences between the BUD/FM pMDI and BUD groups were smaller in patients with mild to moderate asthma than in those with moderate to severe asthma, most likely because patients with milder disease had lower asthma event rates. Overall, these findings support the efficacy of BUD/FM pMDI in achieving asthma control in patients with moderate to severe asthma.
Figures



References
-
- American Lung Association. Trends in asthma morbidity and mortality. 2011.
-
- Nathan RA, Nolte H, Pearlman DS. P04334 Study Investigators. Twenty-six-week efficacy and safety study of mometasone furoate/formoterol 200/10 μg combination treatment in patients with persistent asthma previously receiving medium-dose inhaled corticosteroids. Allergy Asthma Proc. 2010;31(4):269–79. doi: 10.2500/aap.2010.31.3364. - DOI - PubMed

