Questioning the questions that have been asked about the infant brain using near-infrared spectroscopy
- PMID: 22329690
- PMCID: PMC3461095
- DOI: 10.1080/02643294.2012.654773
Questioning the questions that have been asked about the infant brain using near-infrared spectroscopy
Abstract
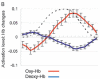
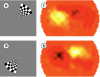
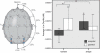
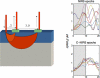
Near-infrared spectroscopy (NIRS) is a noninvasive diffuse optical-imaging technique that can measure local metabolic demand in the surface of the cortex due to differential absorption of light by oxygenated and deoxygenated blood. Over the past decade, NIRS has become increasingly used as a complement to other neuroimaging techniques, such as electroencephalography (EEG), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI), particularly in paediatric populations who cannot easily be tested using fMRI and MEG. In this review of empirical findings from human infants, ranging in age from birth to 12 months of age, a number of interpretive concerns are raised about what can be concluded from NIRS data. In addition, inconsistencies across studies are highlighted, and strategies are proposed for enhancing the reliability of NIRS data gathered from infants. Finally, a variety of new and promising advances in NIRS techniques are highlighted.
Figures




References
-
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Konstantino M, Sarofin H, Schneider KC, Gore JC, Ment LR. Neonatal Auditory Activation Detected by Functional Magnetic Resonance Imaging. Magnetic Resonance Imaging. 2001;19:1–5. - PubMed
-
- Aoyama S, Toshima T, Saito Y, Konishi N, Motoshige K, Ishikawa N, Nakamura K, Kobayashi M. Maternal breast milk odour induces frontal lobe activation in neonates: A NIRS study. Early Human Development. 2010;86:541–545. - PubMed
-
- Ashby FG. Statistical Analysis of fMRI Data. MIT Press; Cambridge. MA: 2011.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
