Aqueous phase separation as a possible route to compartmentalization of biological molecules
- PMID: 22330132
- PMCID: PMC3525015
- DOI: 10.1021/ar200294y
Aqueous phase separation as a possible route to compartmentalization of biological molecules
Abstract
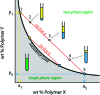
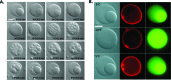
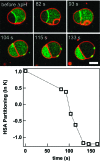
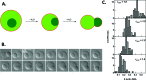
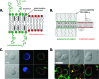
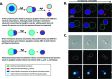
How could the incredible complexity of modern cells evolve from something simple enough to have appeared in a primordial soup? This enduring question has sparked the interest of researchers since Darwin first considered his theory of natural selection. Organic molecules, even potentially functional molecules including peptides and nucleotides, can be produced abiotically. Amphiphiles such as surfactants and lipids display remarkable self-assembly processes including the spontaneous formation of vesicles resembling the membranes of living cells. Nonetheless, numerous questions remain. Given the presumably dilute concentrations of macromolecules in the prebiotic pools where the earliest cells are thought to have appeared, how could the necessary components become concentrated and encapsulated within a semipermeable membrane? What would drive the further structural complexity that is a hallmark of modern living systems? The interior of modern cells is subdivided into microcompartments such as the nucleoid of bacteria or the organelles of eukaryotic cells. Even within what at first appears to be a single compartment, for example, the cytoplasm or nucleus, chemical composition is often nonuniform, containing gradients, macromolecular assemblies, and/or liquid droplets. What might the internal structure of intermediate evolutionary forms have looked like? The nonideal aqueous solution chemistry of macromolecules offers an attractive possible answer to these questions. Aqueous polymer solutions will form multiple coexisting thermodynamic phases under a variety of readily accessible conditions. In this Account, we describe aqueous phase separation as a model system for biological compartmentalization in both early and modern cells, with an emphasis on systems that have been encapsulated within a lipid bilayer. We begin with an introduction to aqueous phase separation and discuss how this phenomenon can lead to microcompartmentalization and could facilitate biopolymer encapsulation by partitioning of solutes between the phases. We then describe primitive model cells based on phase separation inside lipid vesicles, which mimic several basic properties of biological cells: microcompartmentation, protein relocalization in response to stimulus, loss of symmetry, and asymmetric vesicle division. We observe these seemingly complex phenomena in the absence of genetic molecules, enzymes, or cellular machinery, and as a result these processes could provide clues to possible intermediates in the early evolution of cell-like assemblies.
Figures


References
-
- Follmann H.; Brownson C. Darwin’s warm little pond revisited: from molecules to the origin of life. Naturwissenschaften 2009, 96, 1265–1292. - PubMed
-
- Pohorille A.; Deamer D. Self-assembly and function of primitive cell membranes. Res. Microbiol. 2009, 160, 449–456. - PubMed
-
- Kurihara K.; Tamura M.; Shohda K.-i.; Toyota T.; Suzuki K.; Sugawara T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem 2011, 3, 775–781. - PubMed
-
- The Origins of Life. Cold Spring Harbor Perspectives in Biology; Deamer D., Szostak J. W., Eds.; Cold Spring Harbor Press: Cold Spring Harbor, NY, 2010; 318 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

