Time-resolved protein nanocrystallography using an X-ray free-electron laser
- PMID: 22330507
- PMCID: PMC3413412
- DOI: 10.1364/OE.20.002706
Time-resolved protein nanocrystallography using an X-ray free-electron laser
Abstract
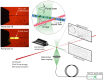
We demonstrate the use of an X-ray free electron laser synchronized with an optical pump laser to obtain X-ray diffraction snapshots from the photoactivated states of large membrane protein complexes in the form of nanocrystals flowing in a liquid jet. Light-induced changes of Photosystem I-Ferredoxin co-crystals were observed at time delays of 5 to 10 µs after excitation. The result correlates with the microsecond kinetics of electron transfer from Photosystem I to ferredoxin. The undocking process that follows the electron transfer leads to large rearrangements in the crystals that will terminally lead to the disintegration of the crystals. We describe the experimental setup and obtain the first time-resolved femtosecond serial X-ray crystallography results from an irreversible photo-chemical reaction at the Linac Coherent Light Source. This technique opens the door to time-resolved structural studies of reaction dynamics in biological systems.
Figures


References
-
- Wöhri A. B., Katona G., Johansson L. C., Fritz E., Malmerberg E., Andersson M., Vincent J., Eklund M., Cammarata M., Wulff M., Davidsson J., Groenhof G., Neutze R., “Light-induced structural changes in a photosynthetic reaction center caught by Laue diffraction,” Science 328(5978), 630–633 (2010).10.1126/science.1186159 - DOI - PubMed
-
- Graber T., Anderson S., Brewer H., Chen Y. S., Cho H. S., Dashdorj N., Henning R. W., Kosheleva I., Macha G., Meron M., Pahl R., Ren Z., Ruan S., Schotte F., Srajer V., Viccaro P. J., Westferro F., Anfinrud P., Moffat K., “BioCARS: a synchrotron resource for time-resolved X-ray science,” J. Synchrotron Radiat. 18(4), 658–670 (2011).10.1107/S0909049511009423 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

