Epigenetic remodelling of brain, body and behaviour during phase change in locusts
- PMID: 22330837
- PMCID: PMC3314403
- DOI: 10.1186/2042-1001-1-11
Epigenetic remodelling of brain, body and behaviour during phase change in locusts
Abstract
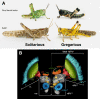
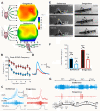
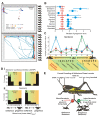
The environment has a central role in shaping developmental trajectories and determining the phenotype so that animals are adapted to the specific conditions they encounter. Epigenetic mechanisms can have many effects, with changes in the nervous and musculoskeletal systems occurring at different rates. How is the function of an animal maintained whilst these transitions happen? Phenotypic plasticity can change the ways in which animals respond to the environment and even how they sense it, particularly in the context of social interactions between members of their own species. In the present article, we review the mechanisms and consequences of phenotypic plasticity by drawing upon the desert locust as an unparalleled model system. Locusts change reversibly between solitarious and gregarious phases that differ dramatically in appearance, general physiology, brain function and structure, and behaviour. Solitarious locusts actively avoid contact with other locusts, but gregarious locusts may live in vast, migrating swarms dominated by competition for scarce resources and interactions with other locusts. Different phase traits change at different rates: some behaviours take just a few hours, colouration takes a lifetime and the muscles and skeleton take several generations. The behavioural demands of group living are reflected in gregarious locusts having substantially larger brains with increased space devoted to higher processing. Phase differences are also apparent in the functioning of identified neurons and circuits. The whole transformation process of phase change pivots on the initial and rapid behavioural decision of whether or not to join with other locusts. The resulting positive feedback loops from the presence or absence of other locusts drives the process to completion. Phase change is accompanied by dramatic changes in neurochemistry, but only serotonin shows a substantial increase during the critical one- to four-hour window during which gregarious behaviour is established. Blocking the action of serotonin or its synthesis prevents the establishment of gregarious behaviour. Applying serotonin or its agonists promotes the acquisition of gregarious behaviour even in a locust that has never encountered another locust. The analysis of phase change in locusts provides insights into a feedback circuit between the environment and epigenetic mechanisms and more generally into the neurobiology of social interaction.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

