Ymer acts as a multifunctional regulator in nuclear factor-κB and Fas signaling pathways
- PMID: 22331027
- PMCID: PMC3388135
- DOI: 10.2119/molmed.2011.00435
Ymer acts as a multifunctional regulator in nuclear factor-κB and Fas signaling pathways
Abstract
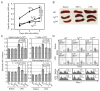
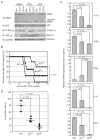
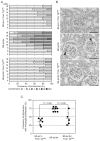
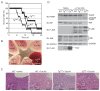
The nuclear factor (NF)-κB family of transcription factors regulates diverse cellular functions, including inflammation, oncogenesis and apoptosis. It was reported that A20 plays a critical role in the termination of NF-κB signaling after activation. Previously, we showed that Ymer interacts and collaborates with A20, followed by degradation of receptor-interacting protein (RIP) and attenuation of NF-κB signaling. Here we show the function of Ymer in regulation of several signaling pathways including NF-κB on the basis of results obtained by using Ymer transgenic (Ymer Tg) mice. Ymer Tg mice exhibited impaired immune responses, including NF-κB and mitogen-activated protein kinase (MAPK) activation, cell proliferation and cytokine production, to tumor necrosis factor (TNF)-α, polyI:C or lipopolysaccharide (LPS) stimulation. Ymer Tg mice were more resistant to LPS-induced septic shock than wild-type mice. Transgene of Ymer inhibited the onset of glomerulonephritis in lpr/lpr mice as an autoimmune disease model. In contrast to the inflammatory immune response to LPS, Fas-mediated cell death was strongly induced in liver cells of Ymer Tg mice in which Ymer is abundantly expressed. These findings suggest that Ymer acts as a regulator downstream of several receptors and that Ymer functions as a positive or negative regulator in a signaling pathway-dependent manner.
Figures

References
-
- Hayden MS, Ghosh S. Signaling to NFkappaB. Genes Dev. 2004;18:2195–224. - PubMed
-
- Yaron A, et al. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 1998;396:590–4. - PubMed
-
- Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

