CoREST acts as a positive regulator of Notch signaling in the follicle cells of Drosophila melanogaster
- PMID: 22331351
- PMCID: PMC3283875
- DOI: 10.1242/jcs.089797
CoREST acts as a positive regulator of Notch signaling in the follicle cells of Drosophila melanogaster
Abstract
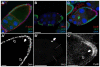
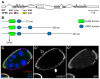
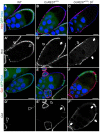
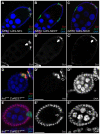
The Notch signaling pathway plays important roles in a variety of developmental events. The context-dependent activities of positive and negative modulators dramatically increase the diversity of cellular responses to Notch signaling. In a screen for mutations affecting the Drosophila melanogaster follicular epithelium, we isolated a mutation in CoREST that disrupts the Notch-dependent mitotic-to-endocycle switch of follicle cells at stage 6 of oogenesis. We show that Drosophila CoREST positively regulates Notch signaling, acting downstream of the proteolytic cleavage of Notch but upstream of Hindsight activity; the Hindsight gene is a Notch target that coordinates responses in the follicle cells. We show that CoREST genetically interacts with components of the Notch repressor complex, Hairless, C-terminal Binding Protein and Groucho. In addition, we demonstrate that levels of H3K27me3 and H4K16 acetylation are dramatically increased in CoREST mutant follicle cells. Our data indicate that CoREST acts as a positive modulator of the Notch pathway in the follicular epithelium as well as in wing tissue, and suggests a previously unidentified role for CoREST in the regulation of Notch signaling. Given its high degree of conservation among species, CoREST probably also functions as a regulator of Notch-dependent cellular events in other organisms.
Figures

Similar articles
-
The First Defined Null Allele of the Notch Regulator, a Suppressor of Deltex: Uncovering Its Novel Roles in Drosophila melanogaster Oogenesis.Biomolecules. 2024 Apr 26;14(5):522. doi: 10.3390/biom14050522. Biomolecules. 2024. PMID: 38785929 Free PMC article.
-
Limited Availability of General Co-Repressors Uncovered in an Overexpression Context during Wing Venation in Drosophila melanogaster.Genes (Basel). 2020 Sep 28;11(10):1141. doi: 10.3390/genes11101141. Genes (Basel). 2020. PMID: 32998295 Free PMC article.
-
Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells.Development. 2001 Dec;128(23):4737-46. doi: 10.1242/dev.128.23.4737. Development. 2001. PMID: 11731454
-
Hairless: the ignored antagonist of the Notch signalling pathway.Hereditas. 2006 Dec;143(2006):212-21. doi: 10.1111/j.2007.0018-0661.01971.x. Hereditas. 2006. PMID: 17362357 Review.
-
Spatio-Temporal Regulation of Notch Activation in Asymmetrically Dividing Sensory Organ Precursor Cells in Drosophila melanogaster Epithelium.Cells. 2024 Jun 30;13(13):1133. doi: 10.3390/cells13131133. Cells. 2024. PMID: 38994985 Free PMC article. Review.
Cited by
-
Distinct CoREST complexes act in a cell-type-specific manner.Nucleic Acids Res. 2019 Dec 16;47(22):11649-11666. doi: 10.1093/nar/gkz1050. Nucleic Acids Res. 2019. PMID: 31701127 Free PMC article.
-
CoREST1 promotes tumor formation and tumor stroma interactions in a mouse model of breast cancer.PLoS One. 2015 Mar 20;10(3):e0121281. doi: 10.1371/journal.pone.0121281. eCollection 2015. PLoS One. 2015. PMID: 25793264 Free PMC article.
-
Oncogenic Notch Triggers Neoplastic Tumorigenesis in a Transition-Zone-like Tissue Microenvironment.Dev Cell. 2019 May 6;49(3):461-472.e5. doi: 10.1016/j.devcel.2019.03.015. Epub 2019 Apr 11. Dev Cell. 2019. PMID: 30982664 Free PMC article.
-
Notch Signaling during Oogenesis in Drosophila melanogaster.Genet Res Int. 2012;2012:648207. doi: 10.1155/2012/648207. Epub 2012 May 3. Genet Res Int. 2012. PMID: 22720165 Free PMC article.
-
Endoreplication and polyploidy: insights into development and disease.Development. 2013 Jan 1;140(1):3-12. doi: 10.1242/dev.080531. Development. 2013. PMID: 23222436 Free PMC article. Review.
References
-
- Artavanis-Tsakonas S., Muskavitch M. A. (2010). Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 92, 1-29 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

