Differential regulation of actin microfilaments by human MICAL proteins
- PMID: 22331357
- PMCID: PMC3367829
- DOI: 10.1242/jcs.089367
Differential regulation of actin microfilaments by human MICAL proteins
Abstract
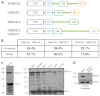
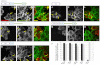
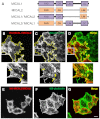
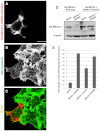
The Drosophila melanogaster MICAL protein is essential for the neuronal growth cone machinery that functions through plexin- and semaphorin-mediated axonal signaling. Drosophila MICAL is also involved in regulating myofilament organization and synaptic structures, and serves as an actin disassembly factor downstream of plexin-mediated axonal repulsion. In mammalian cells there are three known isoforms, MICAL1, MICAL2 and MICAL3, as well as the MICAL-like proteins MICAL-L1 and MICAL-L2, but little is known of their function, and information comes almost exclusively from neural cells. In this study we show that in non-neural cells human MICALs are required for normal actin organization, and all three MICALs regulate actin stress fibers. Moreover, we provide evidence that the generation of reactive oxygen species by MICAL proteins is crucial for their actin-regulatory function. However, although MICAL1 is auto-inhibited by its C-terminal coiled-coil region, MICAL2 remains constitutively active and affects stress fibers. These data suggest differential but complementary roles for MICAL1 and MICAL2 in actin microfilament regulation.
Figures


Similar articles
-
Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly.Cytoskeleton (Hoboken). 2011 Aug;68(8):415-33. doi: 10.1002/cm.20527. Epub 2011 Aug 25. Cytoskeleton (Hoboken). 2011. PMID: 21800438 Free PMC article. Review.
-
MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion.Cell. 2002 Jun 28;109(7):887-900. doi: 10.1016/s0092-8674(02)00794-8. Cell. 2002. PMID: 12110185
-
Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein.J Neurosci. 2008 Feb 27;28(9):2287-97. doi: 10.1523/JNEUROSCI.5646-07.2008. J Neurosci. 2008. PMID: 18305261 Free PMC article.
-
MICAL-like1 mediates epidermal growth factor receptor endocytosis.Mol Biol Cell. 2011 Sep;22(18):3431-41. doi: 10.1091/mbc.E11-01-0030. Epub 2011 Jul 27. Mol Biol Cell. 2011. PMID: 21795389 Free PMC article.
-
MICALs.Curr Biol. 2018 May 7;28(9):R538-R541. doi: 10.1016/j.cub.2018.01.025. Curr Biol. 2018. PMID: 29738722 Free PMC article. Review.
Cited by
-
MICAL2 Facilitates Gastric Cancer Cell Migration via MRTF-A-Mediated CDC42 Activation.Front Mol Biosci. 2021 Mar 24;8:568868. doi: 10.3389/fmolb.2021.568868. eCollection 2021. Front Mol Biosci. 2021. PMID: 33842533 Free PMC article.
-
Comprehensive Analysis of MICALL2 Reveals Its Potential Roles in EGFR Stabilization and Ovarian Cancer Cell Invasion.Int J Mol Sci. 2023 Dec 30;25(1):518. doi: 10.3390/ijms25010518. Int J Mol Sci. 2023. PMID: 38203692 Free PMC article.
-
Characterizing F-actin Disassembly Induced by the Semaphorin-Signaling Component MICAL.Methods Mol Biol. 2017;1493:119-128. doi: 10.1007/978-1-4939-6448-2_8. Methods Mol Biol. 2017. PMID: 27787846 Free PMC article.
-
MICAL2 Contributes to Gastric Cancer Cell Proliferation by Promoting YAP Dephosphorylation and Nuclear Translocation.Oxid Med Cell Longev. 2021 Oct 5;2021:9955717. doi: 10.1155/2021/9955717. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34650666 Free PMC article.
-
Oxidation of F-actin controls the terminal steps of cytokinesis.Nat Commun. 2017 Feb 23;8:14528. doi: 10.1038/ncomms14528. Nat Commun. 2017. PMID: 28230050 Free PMC article.
References
-
- Ashida S., Furihata M., Katagiri T., Tamura K., Anazawa Y., Yoshioka H., Miki T., Fujioka T., Shuin T., Nakamura Y., et al. (2006). Expression of novel molecules, MICAL2-PV (MICAL2 prostate cancer variants), increases with high Gleason score and prostate cancer progression. Clin. Cancer Res. 12, 2767-2773 - PubMed
-
- Beuchle D., Schwarz H., Langegger M., Koch I., Aberle H. (2007). Drosophila MICAL regulates myofilament organization and synaptic structure. Mech. Dev. 124, 390-406 - PubMed
-
- Dalle-Donne I., Rossi R., Giustarini D., Gagliano N., Di Simplicio P., Colombo R., Milzani A. (2002). Methionine oxidation as a major cause of the functional impairment of oxidized actin. Free Radic. Biol. Med. 32, 927-937 - PubMed
-
- Faix J., Breitsprecher D., Stradal T. E., Rottner K. (2009). Filopodia: complex models for simple rods. Int. J. Biochem. Cell Biol. 41, 1656-1664 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

