Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function
- PMID: 22331359
- PMCID: PMC3283876
- DOI: 10.1242/jcs.090639
Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function
Abstract
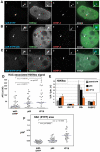
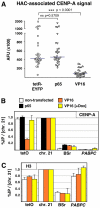
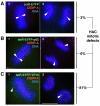
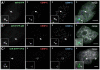
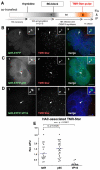
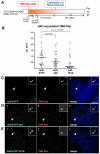
Human kinetochores are transcriptionally active, producing very low levels of transcripts of the underlying alpha-satellite DNA. However, it is not known whether kinetochores can tolerate acetylated chromatin and the levels of transcription that are characteristic of housekeeping genes, or whether kinetochore-associated 'centrochromatin', despite being transcribed at a low level, is essentially a form of repressive chromatin. Here, we have engineered two types of acetylated chromatin within the centromere of a synthetic human artificial chromosome. Tethering a minimal NF-κB p65 activation domain within kinetochore-associated chromatin produced chromatin with high levels of histone H3 acetylated on lysine 9 (H3K9ac) and an ~10-fold elevation in transcript levels, but had no substantial effect on kinetochore assembly or function. By contrast, tethering the herpes virus VP16 activation domain produced similar modifications in the chromatin but resulted in an ~150-fold elevation in transcripts, approaching the level of transcription of an endogenous housekeeping gene. This rapidly inactivated kinetochores, causing a loss of assembled CENP-A and blocking further CENP-A assembly. Our data reveal that functional centromeres in vivo show a remarkable plasticity--kinetochores tolerate profound changes to their chromatin environment, but appear to be critically sensitive to the level of centromeric transcription.
Figures

References
-
- Black B. E., Bassett E. A. (2008). The histone variant CENP-A and centromere specification. Curr. Opin. Cell Biol. 20, 91-100 - PubMed
-
- Black B. E., Foltz D. R., Chakravarthy S., Luger K., Woods V. L., Jr, Cleveland D. W. (2004). Structural determinants for generating centromeric chromatin. Nature 430, 578-882 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

