The origin and composition of cucurbit "phloem" exudate
- PMID: 22331409
- PMCID: PMC3320192
- DOI: 10.1104/pp.112.194431
The origin and composition of cucurbit "phloem" exudate
Abstract
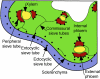
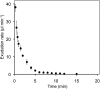
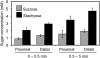
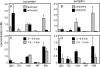
Cucurbits exude profusely when stems or petioles are cut. We conducted studies on pumpkin (Cucurbita maxima) and cucumber (Cucumis sativus) to determine the origin and composition of the exudate. Morphometric analysis indicated that the exudate is too voluminous to derive exclusively from the phloem. Cold, which inhibits phloem transport, did not interfere with exudation. However, ice water applied to the roots, which reduces root pressure, rapidly diminished exudation rate. Sap was seen by microscopic examination to flow primarily from the fascicular phloem in cucumber, and several other cucurbit species, but primarily from the extrafascicular phloem in pumpkin. Following exposure of leaves to 14CO2, radiolabeled stachyose and other sugars were detected in the exudate in proportions expected of authentic phloem sap. Most of this radiolabel was released during the first 20 s. Sugars in exudate were dilute. The sugar composition of exudate from extrafascicular phloem near the edge of the stem differed from that of other sources in that it was high in hexose and low in stachyose. We conclude that sap is released from cucurbit phloem upon wounding but contributes negligibly to total exudate volume. The sap is diluted by water from cut cells, the apoplast, and the xylem. Small amounts of dilute, mobile sap from sieve elements can be obtained, although there is evidence that it is contaminated by the contents of other cell types. The function of P-proteins may be to prevent water loss from the xylem as well as nutrient loss from the phloem.
Figures



Similar articles
-
So similar yet so different: The distinct contributions of extrafascicular and fascicular phloem to transport and exudation in cucumber plants.J Plant Physiol. 2022 Apr;271:153643. doi: 10.1016/j.jplph.2022.153643. Epub 2022 Mar 1. J Plant Physiol. 2022. PMID: 35248933 Review.
-
Comparative proteomics of cucurbit phloem indicates both unique and shared sets of proteins.Plant J. 2016 Nov;88(4):633-647. doi: 10.1111/tpj.13288. Epub 2016 Sep 17. Plant J. 2016. PMID: 27472661
-
Divergent metabolome and proteome suggest functional independence of dual phloem transport systems in cucurbits.Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13532-7. doi: 10.1073/pnas.0910558107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566864 Free PMC article.
-
Interaction of xylem and phloem during exudation and wound occlusion in Cucurbita maxima.Plant Cell Environ. 2013 Jan;36(1):237-47. doi: 10.1111/j.1365-3040.2012.02571.x. Epub 2012 Aug 14. Plant Cell Environ. 2013. PMID: 22765252
-
Review primary and secondary metabolites in phloem sap collected with aphid stylectomy.J Plant Physiol. 2022 Apr;271:153645. doi: 10.1016/j.jplph.2022.153645. Epub 2022 Feb 18. J Plant Physiol. 2022. PMID: 35217406 Review.
Cited by
-
Connecting Source with Sink: The Role of Arabidopsis AAP8 in Phloem Loading of Amino Acids.Plant Physiol. 2016 May;171(1):508-21. doi: 10.1104/pp.16.00244. Epub 2016 Mar 25. Plant Physiol. 2016. PMID: 27016446 Free PMC article.
-
Identification of Long-Distance Mobile mRNAs Responding to Drought Stress in Heterografted Tomato Plants.Int J Mol Sci. 2025 Mar 29;26(7):3168. doi: 10.3390/ijms26073168. Int J Mol Sci. 2025. PMID: 40243940 Free PMC article.
-
The Crucial Roles of Phloem Companion Cells in Response to Phosphorus Deficiency.Plant Cell Environ. 2025 Jun;48(6):4327-4340. doi: 10.1111/pce.15421. Epub 2025 Feb 17. Plant Cell Environ. 2025. PMID: 39960032 Free PMC article.
-
Microneedle Sensors for Ion Monitoring in Plants. One Step Closer to Smart Agriculture.ACS Sens. 2025 Jul 25;10(7):4771-4784. doi: 10.1021/acssensors.5c01215. Epub 2025 Jul 3. ACS Sens. 2025. PMID: 40605515 Free PMC article. Review.
-
Elucidation of the Mechanisms of Long-Distance mRNA Movement in a Nicotiana benthamiana/Tomato Heterograft System.Plant Physiol. 2018 Jun;177(2):745-758. doi: 10.1104/pp.17.01836. Epub 2018 May 2. Plant Physiol. 2018. PMID: 29720554 Free PMC article.
References
-
- Dinant S, Bonnemain JL, Girousse C, Kehr J. (2010) Phloem sap intricacy and interplay with aphid feeding. C R Biol 333: 504–515 - PubMed
-
- Epstein E, Bloom AJ. (2005) Mineral Nutrition of Plants: Principles and Perspectives, Ed 2. Sinauer Associates, Sunderland, MA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

