MacroH2A1 regulates the balance between self-renewal and differentiation commitment in embryonic and adult stem cells
- PMID: 22331466
- PMCID: PMC3318583
- DOI: 10.1128/MCB.06323-11
MacroH2A1 regulates the balance between self-renewal and differentiation commitment in embryonic and adult stem cells
Abstract
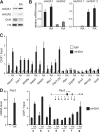
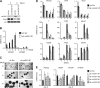
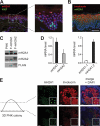
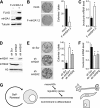
One of the most striking epigenetic alterations that occurs at the level of the nucleosome is the complete exchange of the canonical H2A histones for the macroH2A variant. Here, we provide insight into the poorly recognized function of macroH2A in transcriptional activation and demonstrate its relevance in embryonic and adult stem cells. Knockdown of macroH2A1 in mouse embryonic stem (mES) cells limited their capacity to differentiate but not their self-renewal. The loss of macroH2A1 interfered with the proper activation of differentiation genes, most of which are direct target genes of macroH2A. Additionally, macroH2A1-deficient mES cells displayed incomplete inactivation of pluripotency genes and formed defective embryoid bodies. In vivo, macroH2A1-deficient teratomas contained a massive expansion of malignant, undifferentiated carcinoma tissue. In the heterogeneous culture of primary human keratinocytes, macroH2A1 levels negatively correlated with the self-renewal capacity of the pluripotent compartment. Together these results establish macroH2A1 as a critical chromatin component that regulates the delicate balance between self-renewal and differentiation of embryonic and adult stem cells.
Figures



References
-
- Barde I, Salmon P, Trono D. 2010. Production and titration of lentiviral vectors. Curr. Protoc. Neurosci. 53:4.21.1–4.21.23. - PubMed
-
- Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. 2009. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat. Biotechnol. 27:281–287 - PubMed
-
- Boix R, et al. 2009. Primary renal cell carcinoma in a transplanted kidney: genetic evidence of recipient origin. Transplantation 87:1057–1061 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
